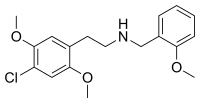
25C-NBOMe
25C-NBOMe (NBOMe-2C-C, 2C-C-NBOMe, Cimbi-82) is a psychedelic drug and derivative of the psychedelic phenethylamine 2C-C. 25C-NBOMe appeared on online vendor sites in 2010 but was not reported in the literature until 2011.[2] It acts as a potent agonist of the 5HT2A receptor,[3] and has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET).[2][4] Multiple deaths have occurred from usage of 25C-NBOMe due to the ease of accidental overdose. The long-term toxic effects of the drug have not been researched.
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H22ClNO3 |
| Molar mass | 335.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

History
25C-NBOMe is derived from the psychedelic phenethylamine 2C-C by substitution on the amine with a 2-methoxybenzyl group. 25C-NBOMe is a clumpy white powder with a notably bitter and metallic taste. 25C-NBOMe has been found on blotter mimics sold as LSD.[5]
Dosage
25C-NBOMe is extremely potent and the effects of the drug increase greatly within a small window of dosage adjustment. Overdose may occur at as little as double an average dose. With inaccurate dosing of street blotter paper, when mistaken for LSD, or when taken as a powder or liquid, this has resulted in multiple accidental deaths.[6]
One study has shown that 25C-NBOMe blotters have 'hotspots' of the drug and the dosage is not evenly applied over the surface of the paper, which could lead to overdose.[7] Sublingually, the threshold for the onset of hallucinogenic effects reportedly is about 100–250 μg, with mild effects at 250–450, strong effects at 450–800, and very strong effects over 800 μg.[8]
NBOMe-substituted compounds have a diminished absorption rate passing through mucus membranes, but generally remain inactive when taken orally. Buccal, sublingual or insufflated routes of administration are all viable options. Absorption rate buccally and sublingually can be increased when complexed with HPBCD complexing sugar, however the most efficient is nasal administration, which shortens the duration while increasing intensity, but has been attributed to several overdoses and deaths.[9]
Effects
Desired
|
Neutral
|
Undesired(Includes negative side effects arising from overdose; likelihood of negative side effects increases with dose)
|
Toxicity and harm potential
NBOMe compounds are often associated with life-threatening toxicity and death.[11][12] Studies on NBOMe family of compounds demonstrated that the substance exhibit neurotoxic and cardiotoxic activity.[13] Reports of autonomic dysfunction remains prevalent with NBOMe compounds, with most individuals experiencing sympathomimetic toxicity such as vasoconstriction, hypertension and tachycardia in addition to hallucinations.[14][15][16][17][18] Other symptoms of toxidrome of include agitation or aggression, seizure, hyperthermia, diaphoresis, hypertonia, rhabdomyolysis, and death.[14][18][12] Researchers report that NBOMe intoxication frequently display signs of serotonin syndrome.[19] The likelihood of seizure is higher in NBOMes compared to other psychedelics.[13]
NBOMe and NBOHs are regularly sold as LSD in blotter papers,[12][20] which have a bitter taste and different safety profiles.[14][11] Despite high potency, recreational doses of LSD have only produced low incidents of acute toxicity.[11] Fatalities involved in NBOMe intoxication suggest that a significant number of individuals ingested the substance which they believed was LSD,[16] and researchers report that "users familiar with LSD may have a false sense of security when ingesting NBOMe inadvertently".[14] While most fatalities are due to the physical effects of the drug, there have also been reports of death due to self-harm and suicide under the influence of the substance.[21][22][14]
Given limited documentation of NBOMe consumption, the long-term effects of the substance remain unknown.[14] NBOMe compounds are not active orally,[lower-alpha 1] and are usually taken sublingually.[24]: 3 When NBOMes are administered sublingually, numbness of the tongue and mouth followed by a metallic chemical taste was observed, and researchers describe this physical side effect as one of the main discriminants between NBOMe compounds and LSD.[25][26][27]Neurotoxic and cardiotoxic actions
Many of the NBOMe compounds have high potency agonist activity at additional 5-HT receptors and prolonged activation of 5-HT2B can cause cardiac valvulopathy in high doses and chronic use.[12][17] 5-HT2B receptors have been strongly implicated in causing drug-induced valvular heart disease.[28][29][30] The high affinity of NBOMe compounds for adrenergic α1 receptor has been reported to contribute to the stimulant-type cardiovascular effects.[17]
In vitro studies, 25C-NBOMe has been shown to exhibit cytotoxicity on neuronal cell lines SH-SY5Y, PC12, and SN471, and the compound was more potent than methamphetamine at reducing the visibility of the respective cells; the neurotoxicity of the compound involves activation of MAPK/ERK cascade and inhibition of Akt/PKB signaling pathway.[13] 25C-NBOMe, including the other derivative 25D-NBOMe, reduced the visibility of cardiomyocytes H9c2 cells, and both substances downregulated expression level of p21 (CDC24/RAC)-activated kinase 1 (PAK1), an enzyme with documented cardiac protective effects.[13]
Preliminary studies on 25C-NBOMe have shown that the substance is toxic to development, heart health, and brain health in zebrafish, rats, and Artemia salina, a common organism for studying potential drug effects on humans, but more research is needed on the topic, the dosages, and if the toxicology results apply to humans. Researchers of the study also recommended further investigation of the drug's potential in damaging pregnant women and their fetus due to the substance's damaging effects to development.[31][32]Emergency treatment
Drug prohibition laws
Canada
As of October 31, 2016; 25C-NBOMe is a controlled substance (Schedule III) in Canada.[33]
New Zealand
25C-NBOMe was sold as a designer drug in New Zealand in early 2012, but was withdrawn from sale after a statement by Associate Health Minister Peter Dunne that 25C-NBOMe would be considered to be substantially similar in chemical structure to the illegal hallucinogen DOB, and was therefore a Class C controlled drug analogue.[36]
Russia
Russia became the first country to regulate the NBOME class. The entire NBOMe series of psychoactives became controlled in the Russian Federation starting October, 2011.[34][37]
Sweden
Sveriges riksdag added 25C-NBOMe to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Aug 1, 2013, published by Medical Products Agency in their regulation LVFS 2013:15 listed as 25C-NBOMe 2-(4-kloro-2,5-dimetoxifenyl)-N-(2-metoxibensyl)etanamin.[38]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[39]
United States
Several NBOMe series compounds will be temporarily scheduled in the United States for 2 years. The temporary scheduling applies to 25C-NBOMe, 25B-NBOMe, and 25I-NBOMe.[40] In November 2015, the temporary scheduling was extended for another year.[41]
China
As of October 2015 25C-NBOMe is a controlled substance in China.[42]
Czech Republic
25C-NBOMe is banned in the Czech Republic.[43]
Analogues and derivatives
Analogues and derivatives of 2C-C:
25C-NB*:
- 25C-NBF
- 25C-NBMD
- 25C-NBOH
- 25C-NBOMe (NBOMe-2CC)
- 25C-NB3OMe
- 25C-NB4OMe
- N-(2C-C)-fentanyl[44]
Notes
- The potency of N-benzylphenethylamines via buccal, sublingual, or nasal absorption is 50-100 greater (by weight) than oral route compared to the parent 2C-x compounds.[23] Researchers hypothesize the low oral metabolic stability of N-benzylphenethylamines is likely causing the low bioavailability on the oral route, although the metabolic profile of this compounds remains unpredictable; therefore researchers state that the fatalities linked to these substances may partly be explained by differences in the metabolism between individuals.[23]
References
- Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–693. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–249. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- Zuba D, Sekuła K, Buczek A (April 2013). "25C-NBOMe--new potent hallucinogenic substance identified on the drug market". Forensic Science International. 227 (1–3): 7–14. doi:10.1016/j.forsciint.2012.08.027. PMID 22989597.
- Kamińska K, Świt P, Malek K (2020). "25C-NBOMe short characterisation". Forensic Toxicology. 38 (2): 490–495. doi:10.1007/s11419-020-00530-1. S2CID 214704393.
- Lützen E, Holtkamp M, Stamme I, Schmid R, Sperling M, Pütz M, Karst U (April 2020). "Multimodal imaging of hallucinogens 25C- and 25I-NBOMe on blotter papers". Drug Testing and Analysis. 12 (4): 465–471. doi:10.1002/dta.2751. PMID 31846172. S2CID 209388281.
- 2C-C-NBOMe Dose - erowid
- Grautoff S, Kähler J (May 2014). "[Near fatal intoxication with the novel psychoactive substance 25C-NBOMe]". Medizinische Klinik, Intensivmedizin und Notfallmedizin (in German). 109 (4): 271–275. doi:10.1007/s00063-014-0360-5. PMID 24770890.
- Jolanta Z, Monika K, and Piotr A (26 February 2020). "NBOMes–Highly Potent and Toxic Alternatives of LSD". Frontiers in Neuroscience. 14: 1-12. doi:10.3389/fnins.2020.00078. PMID 32174803.
- Sean I, Joe R, Jennifer S, and Shaun G (28 March 2022). "A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)". Clinical Toxicology. 60 (8): 966–969. doi:10.1080/15563650.2022.2053150. PMID 35343858. S2CID 247764056.
- Amy E, Katherine W, John R, Sonyoung K, Robert J, Aaron J (December 2018). "Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors". Biochemical Pharmacology. 158: 27–34. doi:10.1016/j.bcp.2018.09.024. PMC 6298744. PMID 30261175.
- Jolanta Z, Monika K, and Piotr A (26 February 2020). "NBOMes–Highly Potent and Toxic Alternatives of LSD". Frontiers in Neuroscience. 14: 78. doi:10.3389/fnins.2020.00078. PMC 7054380. PMID 32174803.
- Lipow M, Kaleem SZ, Espiridion E (30 March 2022). "NBOMe Toxicity and Fatalities: A Review of the Literature". Transformative Medicine. 1 (1): 12–18. doi:10.54299/tmed/msot8578. ISSN 2831-8978. S2CID 247888583.
- Micaela T, Sabrine B, Raffaella A, Giorgia C, Beatrice M, Tatiana B, Federica B, Giovanni S, Francesco B, Fabio G, Krystyna G, Matteo M (21 April 2022). "Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs". Front Psychiatry. 13: 875722. doi:10.3389/fpsyt.2022.875722. PMC 9069068. PMID 35530025.
- Cristina M, Matteo M, Nicholas P, Maria C, Micaela T, Raffaella A, Maria L (12 December 2019). "Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe". Frontiers in Pharmacology. 10: 1406. doi:10.3389/fphar.2019.01406. PMC 6921684. PMID 31915427.
- Anna R, Dino L, Julia R, Daniele B, Marius H, Matthias L (December 2015). "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)". Neuropharmacology. 99: 546–553. doi:10.1016/j.neuropharm.2015.08.034. ISSN 1873-7064. PMID 26318099. S2CID 10382311.
- David W, Roumen S, Andrew C, Paul D (6 February 2015). "Prevalence of use and acute toxicity associated with the use of NBOMe drugs". Clinical Toxicology. 53 (2): 85–92. doi:10.3109/15563650.2015.1004179. PMID 25658166. S2CID 25752763.
- Humston C, Miketic R, Moon K, Ma P, Tobias J (2017-06-05). "Toxic Leukoencephalopathy in a Teenager Caused by the Recreational Ingestion of 25I-NBOMe: A Case Report and Review of Literature". Journal of Medical Cases. 8 (6): 174–179. doi:10.14740/jmc2811w. ISSN 1923-4163.
- Justin P, Stephen R, Kylin A, Alphonse P, Michelle P (2015). "Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper". Journal of Analytical Toxicology. 39 (8): 617–623. doi:10.1093/jat/bkv073. PMC 4570937. PMID 26378135.
- Morini L, Bernini M, Vezzoli S, Restori M, Moretti M, Crenna S, et al. (October 2017). "Death after 25C-NBOMe and 25H-NBOMe consumption". Forensic Science International. 279: e1–e6. doi:10.1016/j.forsciint.2017.08.028. PMID 28893436.
- Byard RW, Cox M, Stockham P (November 2016). "Blunt Craniofacial Trauma as a Manifestation of Excited Delirium Caused by New Psychoactive Substances". Journal of Forensic Sciences. 61 (6): 1546–1548. doi:10.1111/1556-4029.13212. PMID 27723094. S2CID 4734566.
- Sabastian LP, Christoffer B, Martin H, Martin AC, Jan K, Jesper LK (14 February 2014). "Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability". Neurochemical Research. 39 (10): 2018–2023. doi:10.1007/s11064-014-1253-y. PMID 24519542. S2CID 254857910.
- Adam H (18 January 2017). "Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens". Neuropharmacology of New Psychoactive Substances. Current Topics in Behavioral Neurosciences. Vol. 32. Springer. pp. 283–311. doi:10.1007/7854_2016_64. ISBN 978-3-319-52444-3. PMID 28097528.
- Boris D, Cristian C, Marcelo K, Edwar F, Bruce KC (August 2016). "Analysis of 25 C NBOMe in Seized Blotters by HPTLC and GC–MS". Journal of Chromatographic Science. 54 (7): 1153–1158. doi:10.1093/chromsci/bmw095. PMC 4941995. PMID 27406128.
- Francesco SB, Ornella C, Gabriella A, Giuseppe V, Rita S, Flaminia BP, Eduardo C, Pierluigi S, Giovanni M, Guiseppe B, Fabrizio S (3 July 2014). "25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug". BioMed Research International. 2014: 734749. doi:10.1155/2014/734749. PMC 4106087. PMID 25105138.
- Adam JP, Simon HT, Simon LH (September 2021). "Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens". Novel Psychoactive Substances: Classification, Pharmacology and Toxicology (2 ed.). Academic Press. pp. 279–300. doi:10.1016/B978-0-12-818788-3.00008-5. ISBN 978-0-12-818788-3. S2CID 240583877.
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL (Dec 2000). "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications". Circulation. 102 (23): 2836–41. doi:10.1161/01.CIR.102.23.2836. PMID 11104741.
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW (Jan 2000). "Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine". Molecular Pharmacology. 57 (1): 75–81. PMID 10617681.
- Roth BL (Jan 2007). "Drugs and valvular heart disease". The New England Journal of Medicine. 356 (1): 6–9. doi:10.1056/NEJMp068265. PMID 17202450.
- Xu P, Qiu Q, Li H, Yan S, Yang M, Naman CB, et al. (26 February 2019). "25C-NBOMe, a Novel Designer Psychedelic, Induces Neurotoxicity 50 Times More Potent Than Methamphetamine In Vitro". Neurotoxicity Research. 35 (4): 993–998. doi:10.1007/s12640-019-0012-x. PMID 30806983. S2CID 255763701.
- Álvarez-Alarcón N, Osorio-Méndez JJ, Ayala-Fajardo A, Garzón-Méndez WF, Garavito-Aguilar ZV (2021). "Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity". Toxicology Reports. 8: 315–323. doi:10.1016/j.toxrep.2021.01.010. ISSN 2214-7500. PMC 7868744. PMID 33598409.
- "Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". Government of Canada. 4 May 2016. Archived from the original on 5 August 2022. Retrieved 6 May 2023.
- "NBOMe Series Legal Status". Erowid. Retrieved 5 September 2015.
- "Amendment to Dangerous Drugs Ordinance". Israeli Ministry of Health. 7 June 2013. Retrieved 11 September 2015.
- 'Legal high' DIME not so legal. Science Media Centre, March 13th 2012
- "Постановление Правительства Российской Федерации от 6 октября 2011 г. N 822 г. Москва" (in Russian). 19 October 2011. Retrieved 5 September 2015.
- Åkerman CR (24 July 2013). "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika" (PDF). Retrieved 5 September 2015.
- "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.
- Harrigan TM (10 October 2013). "Proposed Rules" (PDF). Drug Enforcement Administration (DEA). Retrieved 5 September 2015.
- Drug Enforcement Administration (November 2015). "Schedules of Controlled Substances: Extension of Temporary Placement of Three Synthetic Phenethylamines in Schedule I. Final order". Federal Register. 80 (219): 70657–70659. PMID 26567439.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví. Archived from the original (PDF) on 2016-03-09. Retrieved 2016-02-06.
- "Explore N-(2C-C)-Fentanyl | PiHKAL · info". isomerdesign.com.