Lubazodone
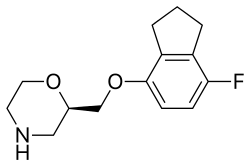
Lubazodone (developmental code names YM-992, YM-35995) is an experimental antidepressant which was under development by Yamanouchi for the treatment for major depressive disorder in the late 1990s and early 2000s but was never marketed.[1][2][3] It acts as a serotonin reuptake inhibitor (Ki for SERTTooltip serotonin transporter = 21 nM) and 5-HT2A receptor antagonist (Ki = 86 nM), and hence has the profile of a serotonin antagonist and reuptake inhibitor (SARI).[1][2] The drug has good selectivity against a range of other monoamine receptors, with its next highest affinities being for the α1-adrenergic receptor (Ki = 200 nM) and the 5-HT2C receptor (Ki = 680 nM).[1] Lubazodone is structurally related to trazodone and nefazodone, but is a stronger serotonin reuptake inhibitor and weaker as a 5-HT2A receptor antagonist in comparison to them and is more balanced in its actions as a SARI.[1][2] It reached phase II clinical trials for depression,[3] but development was discontinued in 2001 reportedly due to the "erosion of the SSRITooltip selective serotonin reuptake inhibitor market in the United States".[1]
 | |
| Clinical data | |
|---|---|
| Other names | YM-992; YM-35995 |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H18FNO2 |
| Molar mass | 251.301 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Moltzen EK, Bang-Andersen B (2006). "Serotonin reuptake inhibitors: the corner stone in treatment of depression for half a century--a medicinal chemistry survey". Curr Top Med Chem. 6 (17): 1801–23. doi:10.2174/156802606778249810. PMID 17017959.
- Zoran Rankovic; Richard Hargreaves; Matilda Bingham (8 October 2012). Drug Discovery for Psychiatric Disorders. Royal Society of Chemistry. pp. 193–. ISBN 978-1-84973-494-3.
- "Lubazodone - AdisInsight".
External links
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||