Amiflamine
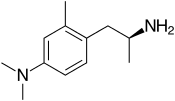
Amiflamine (FLA-336) is a reversible inhibitor of monoamine oxidase A (MAO-A), thereby being a RIMA, and, to a lesser extent, semicarbazide-sensitive amine oxidase (SSAO), as well as a serotonin releasing agent (SRA).[1][2][3][4] It is a derivative of the phenethylamine and amphetamine chemical classes.[1] The (+)-enantiomer is the active stereoisomer.[2]
 | |
| Clinical data | |
|---|---|
| Other names | (+)-4-(dimethylamino)-α,2-dimethylphenethylamine |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H20N2 |
| Molar mass | 192.306 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Amiflamine shows preference for inhibiting MAO-A in serotonergic relative to noradrenergic and dopaminergic neurons.[5][6] In other words, at low doses, it can be used to selectively inhibit MAO-A enzymes in serotonin cells, whereas at higher doses it loses its selectivity.[5][6] This property is attributed to amiflamine's higher affinity for the serotonin transporter over the norepinephrine and dopamine transporters, as transporter-mediated carriage is required for amiflamine to enter monoaminergic neurons.[6]
See also
- Reversible inhibitor of MAO-A (RIMA)
- Monoamine oxidase inhibitor (MAOI)
References
- Ask AL, Högberg K, Schmidt L, Kiessling H, Ross SB (April 1982). "(+)-4-Dimethylamino-2,alpha-dimethylphenethylamine (FLA 336(+)), a selective inhibitor of the A form of monoamine oxidase in the rat brain". Biochemical Pharmacology. 31 (7): 1401–6. doi:10.1016/0006-2952(82)90035-1. PMID 7092929.
- Fowler CJ, Eriksson M, Thorell G, Magnusson O (October 1984). "Stereoselective inhibition of monoamine oxidase and semicarbazide-sensitive amine oxidase by 4-dimethylamino-2,alpha-dimethylphenethylamine (FLA 336)". Naunyn-Schmiedeberg's Archives of Pharmacology. 327 (4): 279–84. doi:10.1007/bf00506237. PMID 6514012. S2CID 25342831.
- Morikawa F, Ueda T, Arai Y, Kinemuchi H (1986). "Inhibition of monoamine oxidase A-form and semicarbazide-sensitive amine oxidase by selective and reversible monoamine oxidase-A inhibitors, amiflamine and FLA 788(+)". Pharmacology. 32 (1): 38–45. doi:10.1159/000138150. PMID 3945672.
- Ask AL, Fagervall I, Huang RB, Ross SB (June 1989). "Release of 3H-5-hydroxytryptamine by amiflamine and related phenylalkylamines from rat occipital cortex slices". Naunyn-Schmiedeberg's Archives of Pharmacology. 339 (6): 684–9. doi:10.1007/bf00168662. PMID 2770890. S2CID 21817180.
- Fowler CJ, Magnusson O, Ross SB (1984). "Intra- and extraneuronal monoamine oxidase". Blood Vessels. 21 (3): 126–31. doi:10.1159/000158505. PMID 6202347.
- Ask AL, Fagervall I, Ross SB (September 1983). "Selective inhibition of monoamine oxidase in monoaminergic neurons in the rat brain". Naunyn-Schmiedeberg's Archives of Pharmacology. 324 (2): 79–87. doi:10.1007/BF00497011. PMID 6646243. S2CID 403633.