Portal:Minerals
Portal maintenance status: (May 2019)
|
The Minerals Portal
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.
The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks.
The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases.
Some natural solid substances without a definite crystalline structure, such as opal or obsidian, are more properly called mineraloids. If a chemical compound occurs naturally with different crystal structures, each structure is considered a different mineral species. Thus, for example, quartz and stishovite are two different minerals consisting of the same compound, silicon dioxide. (Full article...)
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...)
Selected articles
 Image 1
Image 1 A ruby crystal from Dodoma Region, Tanzania
A ruby crystal from Dodoma Region, Tanzania
A ruby is a pinkish red to blood-red colored gemstone, a variety of the mineral corundum (aluminium oxide). Ruby is one of the most popular traditional jewelry gems and is very durable. Other varieties of gem-quality corundum are called sapphires. Ruby is one of the traditional cardinal gems, alongside amethyst, sapphire, emerald, and diamond. The word ruby comes from ruber, Latin for red. The color of a ruby is due to the element chromium.
Some gemstones that are popularly or historically called rubies, such as the Black Prince's Ruby in the British Imperial State Crown, are actually spinels. These were once known as "Balas rubies".
The quality of a ruby is determined by its color, cut, and clarity, which, along with carat weight, affect its value. The brightest and most valuable shade of red, called blood-red or pigeon blood, commands a large premium over other rubies of similar quality. After color follows clarity: similar to diamonds, a clear stone will command a premium, but a ruby without any needle-like rutile inclusions may indicate that the stone has been treated. Ruby is the traditional birthstone for July and is usually pinker than garnet, although some rhodolite garnets have a similar pinkish hue to most rubies. The world's most valuable ruby to be sold at auction is the Sunrise Ruby. (Full article...) Image 2
Image 2
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison.
Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. (Full article...) Image 3
Image 3 Graphite specimen
Graphite specimen
Graphite (/ˈɡræfaɪt/) is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a good (but not excellent) conductor of both heat and electricity. (Full article...) Image 4
Image 4 The slightly misshapen octahedral shape of this rough diamond crystal in matrix is typical of the mineral. Its lustrous faces also indicate that this crystal is from a primary deposit.
The slightly misshapen octahedral shape of this rough diamond crystal in matrix is typical of the mineral. Its lustrous faces also indicate that this crystal is from a primary deposit.
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.
Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high refractive index and a relatively high optical dispersion.
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between 150 and 250 kilometres (93 and 155 mi) in the Earth's mantle, although a few have come from as deep as 800 kilometres (500 mi). Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in volcanic eruptions and deposited in igneous rocks known as kimberlites and lamproites.
Synthetic diamonds can be grown from high-purity carbon under high pressures and temperatures or from hydrocarbon gases by chemical vapor deposition (CVD). Imitation diamonds can also be made out of materials such as cubic zirconia and silicon carbide. Natural, synthetic, and imitation diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements. (Full article...) Image 5
Image 5
Kaolinite (/ˈkeɪ.ələˌnaɪt, -lɪ-/ KAY-ə-lə-nete, -lih-; also called kaolin) is a clay mineral, with the chemical composition Al2Si2O5(OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica (SiO4) linked through oxygen atoms to one octahedral sheet of alumina (AlO6) octahedra.
Kaolinite is a soft, earthy, usually white, mineral (dioctahedral phyllosilicate clay), produced by the chemical weathering of aluminium silicate minerals like feldspar. It has a low shrink–swell capacity and a low cation-exchange capacity (1–15 meq/100 g).
Rocks that are rich in kaolinite, and halloysite, are known as kaolin (/ˈkeɪ.əlɪn/) or china clay. In many parts of the world kaolin is colored pink-orange-red by iron oxide, giving it a distinct rust hue. Lower concentrations yield white, yellow, or light orange colors. Alternating layers are sometimes found, as at Providence Canyon State Park in Georgia, United States.
Kaolin is an important raw material in many industries and applications. Commercial grades of kaolin are supplied and transported as powder, lumps, semi-dried noodle or slurry. Global production of kaolin in 2021 was estimated to be 45 million tonnes, with a total market value of $US4.24 billion. (Full article...) Image 6
Image 6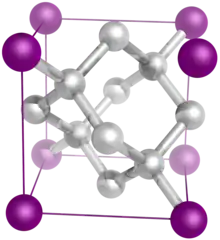 The diamond crystal structure belongs to the face-centered cubic lattice, with a repeated two-atom pattern.
The diamond crystal structure belongs to the face-centered cubic lattice, with a repeated two-atom pattern.
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family.
The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (albeit there are many exceptions). (Full article...) Image 7
Image 7
Chalcopyrite (/ˌkælkəˈpaɪˌraɪt, -koʊ-/ KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.
On exposure to air, chalcopyrite tarnishes to a variety of oxides, hydroxides, and sulfates. Associated copper minerals include the sulfides bornite (Cu5FeS4), chalcocite (Cu2S), covellite (CuS), digenite (Cu9S5); carbonates such as malachite and azurite, and rarely oxides such as cuprite (Cu2O). It is rarely found in association with native copper. Chalcopyrite is a conductor of electricity.
Copper can be extracted from chalcopyrite ore using various methods. The two predominant methods are pyrometallurgy and hydrometallurgy, the former being the most commercially viable. (Full article...) Image 8
Image 8.jpg.webp)
Micas (/ˈmaɪkəz/ MY-kəz) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmatites, and schists, and "books" (large individual crystals) of mica several feet across have been found in some pegmatites.
Micas are used in products such as drywalls, paints, fillers, especially in parts for automobiles, roofing and shingles, as well as in electronics. The mineral is used in cosmetics and food to add "shimmer" or "frost." (Full article...) Image 9
Image 9 Malachite from the Democratic Republic of the Congo
Malachite from the Democratic Republic of the Congo
Malachite is a copper carbonate hydroxide mineral, with the formula Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses, in fractures and deep, underground spaces, where the water table and hydrothermal fluids provide the means for chemical precipitation. Individual crystals are rare, but occur as slender to acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur. (Full article...) Image 10
Image 10 Intergrowth of lustrous, cubic crystals of pyrite, with some surfaces showing characteristic striations, from Huanzala mine, Ancash, Peru. Specimen size: 7.0 × 5.0 × 2.5 cm
Intergrowth of lustrous, cubic crystals of pyrite, with some surfaces showing characteristic striations, from Huanzala mine, Ancash, Peru. Specimen size: 7.0 × 5.0 × 2.5 cm
The mineral pyrite (/ˈpaɪraɪt/ PY-ryte), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula FeS2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of fool's gold. The color has also led to the nicknames brass, brazzle, and brazil, primarily used to refer to pyrite found in coal.
The name pyrite is derived from the Greek πυρίτης λίθος (pyritēs lithos), 'stone or mineral which strikes fire', in turn from πῦρ (pyr), 'fire'. In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what is now called pyrite.
By Georgius Agricola's time, c. 1550, the term had become a generic term for all of the sulfide minerals. (Full article...) Image 11
Image 11 Beachy Head is a part of the extensive Southern England Chalk Formation.
Beachy Head is a part of the extensive Southern England Chalk Formation.
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Chalk is common throughout Western Europe, where deposits underlie parts of France, and steep cliffs are often seen where they meet the sea in places such as the Dover cliffs on the Kent coast of the English Channel.
Chalk is mined for use in industry, such as for quicklime, bricks and builder's putty, and in agriculture, for raising pH in soils with high acidity. It is also used for "blackboard chalk" for writing and drawing on various types of surfaces, although these can also be manufactured from other carbonate-based minerals, or gypsum. (Full article...) Image 12
Image 12
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum.
Rutile derives its name from the Latin rutilus ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality. (Full article...) Image 13
Image 13 A rich seam of iridescent opal encased in matrix
A rich seam of iridescent opal encased in matrix
Opal is a hydrated amorphous form of silica (SiO2·nH2O); its water content may range from 3 to 21% by weight, but is usually between 6 and 10%. Due to its amorphous property, it is classified as a mineraloid, unlike crystalline forms of silica, which are considered minerals. It is deposited at a relatively low temperature and may occur in the fissures of almost any kind of rock, being most commonly found with limonite, sandstone, rhyolite, marl, and basalt.
The name opal is believed to be derived from the Sanskrit word upala (उपल), which means 'jewel', and later the Greek derivative opállios (ὀπάλλιος).
There are two broad classes of opal: precious and common. Precious opal displays play-of-color (iridescence); common opal does not. Play-of-color is defined as "a pseudo chromatic optical effect resulting in flashes of colored light from certain minerals, as they are turned in white light." The internal structure of precious opal causes it to diffract light, resulting in play-of-color. Depending on the conditions in which it formed, opal may be transparent, translucent, or opaque, and the background color may be white, black, or nearly any color of the visual spectrum. Black opal is considered the rarest, while white, gray, and green opals are the most common. (Full article...) Image 14
Image 14 Quartz crystal cluster from Brazil
Quartz crystal cluster from Brazil
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar.
Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at 573 °C (846 K; 1,063 °F). Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold.
There are many different varieties of quartz, several of which are classified as gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia.
Quartz is the mineral defining the value of 7 on the Mohs scale of hardness, a qualitative scratch method for determining the hardness of a material to abrasion. (Full article...) Image 15
Image 15 A sample of andesite (dark groundmass) with amygdaloidal vesicles filled with zeolite. Diameter of view is 8 cm.
A sample of andesite (dark groundmass) with amygdaloidal vesicles filled with zeolite. Diameter of view is 8 cm.
Andesite (/ˈændəzaɪt/) is a volcanic rock of intermediate composition. In a general sense, it is the intermediate type between silica-poor basalt and silica-rich rhyolite. It is fine-grained (aphanitic) to porphyritic in texture, and is composed predominantly of sodium-rich plagioclase plus pyroxene or hornblende.
Andesite is the extrusive equivalent of plutonic diorite. Characteristic of subduction zones, andesite represents the dominant rock type in island arcs. The average composition of the continental crust is andesitic. Along with basalts, andesites are a component of the Martian crust.
The name andesite is derived from the Andes mountain range, where this rock type is found in abundance. It was first applied by Christian Leopold von Buch in 1826. (Full article...) Image 16
Image 16 A crystalline solid: atomic resolution image of strontium titanate. Brighter spots are columns of strontium atoms and darker ones are titanium-oxygen columns.
A crystalline solid: atomic resolution image of strontium titanate. Brighter spots are columns of strontium atoms and darker ones are titanium-oxygen columns.
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word crystallography is derived from the Ancient Greek word κρύσταλλος (krústallos; "clear ice, rock-crystal"), with its meaning extending to all solids with some degree of transparency, and γράφειν (gráphein; "to write"). In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography.
Before the development of X-ray diffraction crystallography (see below), the study of crystals was based on physical measurements of their geometry using a goniometer. This involved measuring the angles of crystal faces relative to each other and to theoretical reference axes (crystallographic axes), and establishing the symmetry of the crystal in question. The position in 3D space of each crystal face is plotted on a stereographic net such as a Wulff net or Lambert net. The pole to each face is plotted on the net. Each point is labelled with its Miller index. The final plot allows the symmetry of the crystal to be established.
Crystallographic methods now depend on analysis of the diffraction patterns of a sample targeted by a beam of some type. X-rays are most commonly used; other beams used include electrons or neutrons. Crystallographers often explicitly state the type of beam used, as in the terms X-ray crystallography, neutron diffraction and electron diffraction. These three types of radiation interact with the specimen in different ways.- X-rays interact with the spatial distribution of electrons in the sample.
- Electrons are charged particles and therefore interact with the total charge distribution of both the atomic nuclei and the electrons of the sample.
- Neutrons are scattered by the atomic nuclei through the strong nuclear forces, but in addition, the magnetic moment of neutrons is non-zero. They are therefore also scattered by magnetic fields. When neutrons are scattered from hydrogen-containing materials, they produce diffraction patterns with high noise levels. However, the material can sometimes be treated to substitute deuterium for hydrogen. Because of these different forms of interaction, the three types of radiation are suitable for different crystallographic studies.
The following sections mainly describe the use of X-rays. See these links for further information on using electrons for diffraction, crystallographic imaging, in transmission, reflection or for surfaces. For addition details on using neutrons see neutron diffraction. (Full article...) Image 17
Image 17
Tourmaline (/ˈtʊərməlɪn, -ˌliːn/ TOOR-mə-lin, -leen) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a wide variety of colors.
The name is derived from the Sinhalese tōramalli (ටෝරමල්ලි), which refers to the carnelian gemstones. (Full article...) Image 18
Image 18
Amethyst is a violet variety of quartz. The name comes from the Koine Greek αμέθυστος amethystos from α- a-, "not" and μεθύσκω (Ancient Greek) methysko / μεθώ metho (Modern Greek), "intoxicate", a reference to the belief that the stone protected its owner from drunkenness. Ancient Greeks wore amethyst and carved drinking vessels from it in the belief that it would prevent intoxication.
Amethyst, a semiprecious stone, is often used in jewelry. (Full article...) Image 19
Image 19 Deep green isolated fluorite crystal resembling a truncated octahedron, set upon a micaceous matrix, from Erongo Mountain, Erongo Region, Namibia (overall size: 50 mm × 27 mm, crystal size: 19 mm wide, 30 g)
Deep green isolated fluorite crystal resembling a truncated octahedron, set upon a micaceous matrix, from Erongo Mountain, Erongo Region, Namibia (overall size: 50 mm × 27 mm, crystal size: 19 mm wide, 30 g)
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite.
Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also usable in the far-ultraviolet and mid-infrared ranges, where conventional glasses are too opaque for use. (Full article...) Image 20
Image 20
Corundum is a crystalline form of aluminium oxide (Al2O3) typically containing traces of iron, titanium, vanadium, and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange.
The name "corundum" is derived from the Tamil-Dravidian word kurundam (ruby-sapphire) (appearing in Sanskrit as kuruvinda).
Because of corundum's hardness (pure corundum is defined to have 9.0 on the Mohs scale), it can scratch almost all other minerals. It is commonly used as an abrasive on sandpaper and on large tools used in machining metals, plastics, and wood. Emery, a variety of corundum with no value as a gemstone, is commonly used as an abrasive. It is a black granular form of corundum, in which the mineral is intimately mixed with magnetite, hematite, or hercynite.
In addition to its hardness, corundum has a density of 4.02 g/cm3 (251 lb/cu ft), which is unusually high for a transparent mineral composed of the low-atomic mass elements aluminium and oxygen. (Full article...) Image 21
Image 21 A rock containing three crystals of pyrite (FeS2). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural crystal facets.
A rock containing three crystals of pyrite (FeS2). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural crystal facets.
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of these crystals:- Primitive cubic (abbreviated cP and alternatively called simple cubic)
- Body-centered cubic (abbreviated cI or bcc)
- Face-centered cubic (abbreviated cF or fcc)
Note: the term fcc is often used in synonym for the cubic close-packed or ccp structure occurring in metals. However, fcc stands for a face-centered-cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed.
Each is subdivided into other variants listed below. Although the unit cells in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. (Full article...) Image 22
Image 22 Green fluorite with prominent cleavage
Green fluorite with prominent cleavage
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage". The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica".
Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral pattern with short covalent bonds. The planes of weakness (cleavage planes) in a diamond are in four directions, following the faces of the octahedron. In graphite, carbon atoms are contained in layers in a hexagonal pattern where the covalent bonds are shorter (and thus even stronger) than those of diamond. However, each layer is connected to the other with a longer and much weaker van der Waals bond. This gives graphite a single direction of cleavage, parallel to the basal pinacoid. So weak is this bond that it is broken with little force, giving graphite a slippery feel as layers shear apart. As a result, graphite makes an excellent dry lubricant.
While all single crystals will show some tendency to split along atomic planes in their crystal structure, if the differences between one direction or another are not large enough, the mineral will not display cleavage. Corundum, for example, displays no cleavage. (Full article...) Image 23
Image 23_-_Kopalnia_soli_Wieliczka%252C_Polska.jpg.webp) Halite from the Wieliczka salt mine, Małopolskie, Poland
Halite from the Wieliczka salt mine, Małopolskie, Poland
Halite (/ˈhælaɪt, ˈheɪlaɪt/), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride (NaCl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals. It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates. The name halite is derived from the Ancient Greek word for "salt", ἅλς (háls). (Full article...) Image 24
Image 24
Talc, or talcum, is a clay mineral composed of hydrated magnesium silicate, with the chemical formula Mg3Si4O10(OH)2. Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant. It is an ingredient in ceramics, paints, and roofing material. It is a main ingredient in many cosmetics. It occurs as foliated to fibrous masses, and in an exceptionally rare crystal form. It has a perfect basal cleavage and an uneven flat fracture, and it is foliated with a two-dimensional platy form.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 1 as the hardness of talc, the softest mineral. When scraped on a streak plate, talc produces a white streak; though this indicator is of little importance, because most silicate minerals produce a white streak. Talc is translucent to opaque, with colors ranging from whitish grey to green with a vitreous and pearly luster. Talc is not soluble in water, and is slightly soluble in dilute mineral acids.
Soapstone is a metamorphic rock composed predominantly of talc. (Full article...) Image 25
Image 25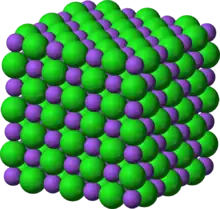 Crystal structure of table salt (sodium in purple, chlorine in green)
Crystal structure of table salt (sodium in purple, chlorine in green)
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.
The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice.
The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called lattice parameters or cell parameters. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space may be described by the 230 space groups.
The crystal structure and symmetry play a critical role in determining many physical properties, such as cleavage, electronic band structure, and optical transparency. (Full article...)
Selected mineralogist
 Image 1
Image 1.jpg.webp) Ernst Friedrich Germar
Ernst Friedrich Germar
Ernst Friedrich Germar (3 November 1786 – 8 July 1853) was a German professor and director of the Mineralogical Museum at Halle. As well as being a mineralogist he was interested in entomology and particularly in the Coleoptera and Hemiptera. He monographed the heteropteran family Scutelleridae.
In 1845, he was elected a foreign member of the Royal Swedish Academy of Sciences. (Full article...) Image 2
Image 2 19th-century illustration of Pliny
19th-century illustration of Pliny
Gaius Plinius Secundus (AD 23/24 – AD 79), called Pliny the Elder (/ˈplɪni/), was a Roman author, naturalist, natural philosopher, and naval and army commander of the early Roman Empire, and a friend of the emperor Vespasian. He wrote the encyclopedic Naturalis Historia (Natural History), which became an editorial model for encyclopedias. He spent most of his spare time studying, writing, and investigating natural and geographic phenomena in the field.
Among Pliny's greatest works was the twenty-volume work Bella Germaniae ("The History of the German Wars"), which is no longer extant. Bella Germaniae, which began where Aufidius Bassus' Libri Belli Germanici ("The War with the Germans") left off, was used as a source by other prominent Roman historians, including Plutarch, Tacitus, and Suetonius. Tacitus may have used Bella Germaniae as the primary source for his work, De origine et situ Germanorum ("On the Origin and Situation of the Germans"). (Full article...) Image 3
Image 3 Henri Longchambon in 1954
Henri Longchambon in 1954
Henri Longchambon (27 July 1896 in Clermont-Ferrand, Puy-de-Dôme – 20 March 1969 in Le Kremlin-Bicêtre) was a French politician and scientist. (Full article...) Image 4
Image 4.png.webp)
Charles Palache (July 18, 1869 – December 5, 1954) was an American mineralogist and crystallographer. In his time, he was one of the most important mineralogists in the United States. (Full article...) Image 5
Image 5 Alexandra Navrotsky with Lee Penn performing the methane mamba chemical demonstration
Alexandra Navrotsky with Lee Penn performing the methane mamba chemical demonstration
Alexandra Navrotsky (born 20 June 1943 in New York City) is a physical chemist in the field of nanogeoscience. She is an elected member of the United States National Academy of Sciences (NAS) and the American Philosophical Society (APS). She was a board member of the Earth Sciences and Resources division of the NAS from 1995 until 2000.
In 2005, she was awarded the Urey Medal, by the European Association of Geochemistry.
In 2006, she was awarded the Harry H. Hess Medal, by the American Geophysical Union.
She is currently the director of NEAT ORU (Nanomaterials in Environment, Agriculture, and Technology Organized Research Unit), a primary program in nanogeoscience. She is distinguished professor at University of California, Davis. (Full article...) Image 6Franz-Joseph Müller, Freiherr von Reichenstein or Franz-Joseph Müller von Reichenstein (1 July 1740 or 4 October 1742 – 12 October 1825 or 1826) was an Austrian mineralogist and mining engineer. Müller held several positions in the Habsburg monarchy administration of mines and coinage in the Banat, Transylvania, and Tyrol. During his time in Transylvania he discovered tellurium in 1782. In his later career he became a member of the imperial council in Vienna and was knighted and elevated to the rank Freiherr in 1820. (Full article...)
Image 6Franz-Joseph Müller, Freiherr von Reichenstein or Franz-Joseph Müller von Reichenstein (1 July 1740 or 4 October 1742 – 12 October 1825 or 1826) was an Austrian mineralogist and mining engineer. Müller held several positions in the Habsburg monarchy administration of mines and coinage in the Banat, Transylvania, and Tyrol. During his time in Transylvania he discovered tellurium in 1782. In his later career he became a member of the imperial council in Vienna and was knighted and elevated to the rank Freiherr in 1820. (Full article...) Image 7Bernard (Bernie) Wood FRS MAE is a British geologist, and professor of mineralogy and senior research fellow at the University of Oxford. He specializes in the thermodynamics of geological systems, using experimental techniques. He is a prominent figure in the field of experimental petrology, having received multiple awards throughout his career and taught at several universities worldwide. (Full article...)
Image 7Bernard (Bernie) Wood FRS MAE is a British geologist, and professor of mineralogy and senior research fellow at the University of Oxford. He specializes in the thermodynamics of geological systems, using experimental techniques. He is a prominent figure in the field of experimental petrology, having received multiple awards throughout his career and taught at several universities worldwide. (Full article...) Image 8
Image 8.jpg.webp) C. E. A. Wichmann
C. E. A. Wichmann
Carl Ernst Arthur Wichmann (9 April 1851 Hamburg – 28 November 1927 Hamburg) was a German geologist and mineralogist. He was professor in geology at Utrecht University from 1879 to 1921, where he founded the geological institute. His daughter was the jurist and anarchist-socialist Clara Wichmann, his son the artist and fascist Erich Wichmann.
Arthur Wichmann spent his youth in Hamburg, where his father ran a boarding school. From 1871 to 1874 he studied at Leipzig University, where he was a pupil of Ferdinand Zirkel, from whom he got his interest in mineralogy. After he spent a few years as assistant to Zirkel he became professor at Utrecht University. (Full article...) Image 9
Image 9
Thomas Egleston (December 9, 1832 – January 15, 1900) was an American engineer who helped found Columbia University's School of Mines, now the Fu Foundation School of Engineering and Applied Science. Throughout his lifetime, Egleston published numerous lectures and books on metallurgy. Many of his books are preserved today at the archive in the Library of Congress. (Full article...) Image 10Walter Frederick Ferrier (1865–1950) was a Canadian geologist and mining engineer.
Image 10Walter Frederick Ferrier (1865–1950) was a Canadian geologist and mining engineer.
He graduated from McGill University's school of mining engineering. He was a tireless mineral collector and was known for walking straight into mining offices to request specimens. Consequently, he created large collections of mineral specimens of a quality still admired to this day. Many classic specimens would never be in collections had it not been for his effort and skill.
The mineral specimens he amassed were instrumental in creating the mineral collections of the Smithsonian in Washington DC, the Royal Ontario Museum in Toronto, Ontario, Canada, University of Alberta, and the museum particularly dear to his heart, the Redpath Museum at McGill University in Montreal, Quebec, Canada. (Full article...) Image 11
Image 11 Johan Gottlieb Gahn
Johan Gottlieb Gahn
Johan Gottlieb Gahn (19 August 1745 – 8 December 1818) was a Swedish chemist and metallurgist who isolated manganese in 1774.
Gahn studied in Uppsala 1762 – 1770 and became acquainted with chemists Torbern Bergman and Carl Wilhelm Scheele. 1770 he settled in Falun, where he introduced improvements in copper smelting, and participated in building up several factories, including those for vitriol, sulfur and red paint. (Full article...) Image 12Johannes Otto Conrad Mügge (4 March 1858, Hannover – 9 June 1932, Göttingen) was a German mineralogist and crystallographer.
Image 12Johannes Otto Conrad Mügge (4 March 1858, Hannover – 9 June 1932, Göttingen) was a German mineralogist and crystallographer.
From 1875 to 1879 he studied mathematics and sciences at the Technical University of Hannover and at the University of Göttingen. After graduation, he spent three years as an assistant to Harry Rosenbusch at the mineralogical-geological institute of the University of Heidelberg. From 1882 he worked as curator of the mineralogical and geological department at the Natural History Museum in Hamburg, and in 1886 became an associate professor at the academy in Münster. Later on, he served as a full professor at the University of Königsberg, where in 1903/04 he was named dean to the faculty of philosophy. In 1908 he relocated as a professor to the University of Göttingen. (Full article...) Image 13Anselmus de Boodt or Anselmus Boetius de Boodt (Bruges, 1550 - Bruges, 21 June 1632) was a Flemish humanist naturalist, Rudolf II physician's gemologist. Along with the German known as Georgius Agricola with mineralogy, de Boodt was responsible for establishing modern gemology. De Boodt was an avid gems and minerals collector who travelled widely to various mining regions in Belgium, Germany, Bohemia and Silesia to collect samples. His definitive work on the subject was the Gemmarum et Lapidum Historia (1609).
Image 13Anselmus de Boodt or Anselmus Boetius de Boodt (Bruges, 1550 - Bruges, 21 June 1632) was a Flemish humanist naturalist, Rudolf II physician's gemologist. Along with the German known as Georgius Agricola with mineralogy, de Boodt was responsible for establishing modern gemology. De Boodt was an avid gems and minerals collector who travelled widely to various mining regions in Belgium, Germany, Bohemia and Silesia to collect samples. His definitive work on the subject was the Gemmarum et Lapidum Historia (1609).
De Boodt was also a gifted draughtsman who made many natural history illustrations and developed a natural history taxonomy. (Full article...) Image 14Jean-André Mongez (21 November 1750 – May 1788) was a French priest and mineralogist. He is presumed to have died at Vanikoro, on the La Pérouse expedition. (Full article...)
Image 14Jean-André Mongez (21 November 1750 – May 1788) was a French priest and mineralogist. He is presumed to have died at Vanikoro, on the La Pérouse expedition. (Full article...) Image 15Gustaf Flink, born 18 January 1848 in Ås Parish, Skaraborg County, died 11 January 1931, was a Swedish mineralogist.
Image 15Gustaf Flink, born 18 January 1848 in Ås Parish, Skaraborg County, died 11 January 1931, was a Swedish mineralogist.
Flink received training as a primary school teacher and graduated in Gothenburg in 1869. In 1871 he received a teaching position in Stockholm. He accompanied Adolf Erik Nordenskiöld on his expedition to Greenland, during which he collected minerals and petrified plants on Iceland in 1883. He returned to Iceland in 1893. On behalf of a Royal Danish geological and geographical commission he made mineralogical investigations in southern Greenland in 1897. (Full article...) Image 16
Image 16.jpg.webp) Johan Afzelius
Johan Afzelius
Johan Afzelius (13 June 1753 in Larv – 20 May 1837 in Uppsala) was a Swedish chemist and notable as the doctoral advisor of one of the founders of modern chemistry, Jöns Jacob Berzelius. He was the brother of botanist Adam Afzelius and physician Pehr von Afzelius.
Afzelius received his PhD at Uppsala University in 1776 under Torbern Olof Bergman. In 1780 he became a lecturer at Uppsala and in 1784 a professor of chemistry. From 1792 to 1797 he undertook research trips to Norway, Denmark and Russia in order to study mineral deposits and to visit scientific institutions. His remarkable mineral collection became part of Uppsala University's mineral cabinet. (Full article...)![Image 17Friedel in 1890sCharles Friedel (French: [fʁidɛl]; 12 March 1832 – 20 April 1899) was a French chemist and mineralogist. (Full article...)](../I/Blank.png.webp) Image 17
Image 17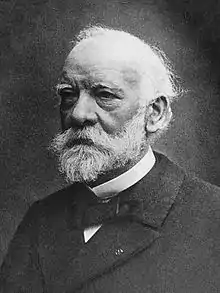 Friedel in 1890s
Friedel in 1890s
Charles Friedel (French: [fʁidɛl]; 12 March 1832 – 20 April 1899) was a French chemist and mineralogist. (Full article...)![Image 18Evgraf Stepanovich FedorovEvgraf Stepanovich Fedorov (Russian: Евгра́ф Степа́нович Фёдоров, 22 December [O.S. 10 December] 1853 – 21 May 1919) was a Russian mathematician, crystallographer and mineralogist.Fedorov was born in the Russian city of Orenburg. His father was a topographical engineer. The family later moved to Saint Petersburg. From the age of fifteen, he was deeply interested in the theory of polytopes, which later became his main research interest. He was a distinguished graduate of the Gorny Institute, which he joined at the age of 26. He was elected the first Director of the Institute in 1905. (Full article...)](../I/Blank.png.webp) Image 18
Image 18 Evgraf Stepanovich Fedorov
Evgraf Stepanovich Fedorov
Evgraf Stepanovich Fedorov (Russian: Евгра́ф Степа́нович Фёдоров, 22 December [O.S. 10 December] 1853 – 21 May 1919) was a Russian mathematician, crystallographer and mineralogist.
Fedorov was born in the Russian city of Orenburg. His father was a topographical engineer. The family later moved to Saint Petersburg. From the age of fifteen, he was deeply interested in the theory of polytopes, which later became his main research interest. He was a distinguished graduate of the Gorny Institute, which he joined at the age of 26. He was elected the first Director of the Institute in 1905. (Full article...) Image 19Leonard James Spencer CBE FRS (7 July 1870 – 14 April 1959) was a British geologist. He was an Honorary member of the Royal Geological Society of Cornwall, and also a recipient of its Bolitho Medal. He was president of the Mineralogical Society of Great Britain and Ireland from 1936 to 1939. In mineralogy Spencer was an original investigator who described several new minerals, including miersite, tarbuttite and parahopeite. He also did important work as a curator, editor and bibliographer. He was the third person to receive the Roebling Medal, the highest award of the Mineralogical Society of America. He wrote at least 146 articles for the Encyclopædia Britannica Eleventh Edition.
Image 19Leonard James Spencer CBE FRS (7 July 1870 – 14 April 1959) was a British geologist. He was an Honorary member of the Royal Geological Society of Cornwall, and also a recipient of its Bolitho Medal. He was president of the Mineralogical Society of Great Britain and Ireland from 1936 to 1939. In mineralogy Spencer was an original investigator who described several new minerals, including miersite, tarbuttite and parahopeite. He also did important work as a curator, editor and bibliographer. He was the third person to receive the Roebling Medal, the highest award of the Mineralogical Society of America. He wrote at least 146 articles for the Encyclopædia Britannica Eleventh Edition.
His daughter, Penelope Spencer became a successful free-style dancer and choreographer. (Full article...) Image 20
Image 20 Teachers of the Forestry Academy in Eberswalde (ca. 1868); Adolf Remelé, 3rd figure from the right (standing).
Teachers of the Forestry Academy in Eberswalde (ca. 1868); Adolf Remelé, 3rd figure from the right (standing).
Adolf Karl Remelé (17 July 1839, Uerdingen – 16 November 1915, Eberswalde) was a German geologist and mineralogist.
He received his education at the University of Bonn, at the École des Mines in Paris and from the University of Berlin, receiving his doctorate in 1864 with the dissertation "De rubro uranico". In 1867 he qualified as a lecturer at Berlin, and during the following year, succeeded Lothar Meyer at the Forestry Academy in Eberswalde, where he taught classes in chemistry, geognosy and mineralogy. (Full article...) Image 21
Image 21 Robert Jameson
Robert Jameson
Robert Jameson FRS FRSE (11 July 1774 – 19 April 1854) was a Scottish naturalist and mineralogist.
As Regius Professor of Natural History at the University of Edinburgh for fifty years, developing his predecessor John Walker's concepts based on mineralogy into geological theories of Neptunism which held sway into the 1830s. Jameson is notable for his advanced scholarship, and his museum collection. The minerals and fossils collection of the Museum of Edinburgh University became one of the largest in Europe during Jameson's long tenure at the university. (Full article...) Image 22Wilhelm Hermann Julius Eitel (6 May 1891, Frankfurt am Main – 20 July 1979, United States) was a German-American scientist. (Full article...)
Image 22Wilhelm Hermann Julius Eitel (6 May 1891, Frankfurt am Main – 20 July 1979, United States) was a German-American scientist. (Full article...) Image 23Naima Sahlbom (15 May 1871 – 29 March 1957) was a Swedish chemist, mineralogist, and peace activist. She is considered to be one of Sweden's most notable women chemists of the early 20th century. (Full article...)
Image 23Naima Sahlbom (15 May 1871 – 29 March 1957) was a Swedish chemist, mineralogist, and peace activist. She is considered to be one of Sweden's most notable women chemists of the early 20th century. (Full article...) Image 24
Image 24 Sigmund Zois by Janez Andrej Herrlein
Sigmund Zois by Janez Andrej Herrlein
Sigmund Zois Freiherr von Edelstein, usually referred as Sigmund Zois (Slovene: Žiga Zois, formerly Slovenized as Cojs or Cojz; pronunciation) (23 November 1747 – 10 November 1819) was a Carniolan nobleman, natural scientist and patron of the arts. He is considered one of the most influential figures of the Enlightenment Era in the Slovene Lands of Habsburg Austria. (Full article...)![Image 25René Just HaüyRené Just Haüy (French pronunciation: [aɥi]) FRS MWS FRSE (28 February 1743 – 1 June 1822) was a French priest and mineralogist, commonly styled the Abbé Haüy after he was made an honorary canon of Notre Dame. Due to his innovative work on crystal structure and his four-volume Traité de Minéralogie (1801),he is often referred to as the "Father of Modern Crystallography". During the French revolution he also helped to establish the metric system. (Full article...)](../I/Blank.png.webp) Image 25
Image 25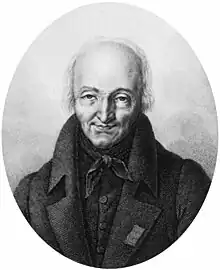 René Just Haüy
René Just Haüy
René Just Haüy (French pronunciation: [aɥi]) FRS MWS FRSE (28 February 1743 – 1 June 1822) was a French priest and mineralogist, commonly styled the Abbé Haüy after he was made an honorary canon of Notre Dame. Due to his innovative work on crystal structure and his four-volume Traité de Minéralogie (1801),
he is often referred to as the "Father of Modern Crystallography". During the French revolution he also helped to establish the metric system. (Full article...)
Related portals
Get involved
For editor resources and to collaborate with other editors on improving Wikipedia's Minerals-related articles, see WikiProject Rocks and minerals.
General images
 Image 1An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral)
Image 1An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral) Image 2Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral)
Image 2Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral) Image 3Gypsum desert rose (from Mineral)
Image 3Gypsum desert rose (from Mineral) Image 4Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
Image 4Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
 Image 6Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral)
Image 6Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral)


 Image 10Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral)
Image 10Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral) Image 11Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral)
Image 11Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral) Image 12Epidote often has a distinctive pistachio-green colour. (from Mineral)
Image 12Epidote often has a distinctive pistachio-green colour. (from Mineral)

 Image 15Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral)
Image 15Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral) Image 16Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral)
Image 16Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral) Image 17Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)
Image 17Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)
 Image 19Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral)
Image 19Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral) Image 20When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
Image 20When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
 Image 22Black andradite, an end-member of the orthosilicate garnet group. (from Mineral)
Image 22Black andradite, an end-member of the orthosilicate garnet group. (from Mineral)
 Image 24Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale)
Image 24Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale) Image 25Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral)
Image 25Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral)
Did you know ...?

- ... that the mineral diaboleite (pictured) was so named out of desperation?
- ... that silicate perovskites may make up to 93% of the lower mantle and that the magnesium form is considered to be Earth's most abundant mineral?
- ... that while huemulite was discovered in 1959, it was not described until 1966?
Subcategories
- Select [►] to view subcategories
Topics
| Overview |  | |
|---|---|---|
| Common minerals | ||
Ore minerals, mineral mixtures and ore deposits | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ores |
| ||||||||
| Deposit types | |||||||||
| Borates | |||||
|---|---|---|---|---|---|
| Carbonates | |||||
| Oxides |
| ||||
| Phosphates | |||||
| Silicates | |||||
| Sulfides | |||||
| Other |
| ||||
| Micas |
|
|---|---|
| Talcs |
|
| Pyrophyllite series |
|
| Kaolinites |
|
| Serpentines |
|
| Corrensites | |
| Smectites and vermiculite family |
|
| Chlorites | |
| Allophanes |
|
| Sepiolites |
|
| Pyrosmalites |
|
| Stilpnomelanes |
|
Structural groups mainly; based on rruff.info/ima, modified | |
| Crystalline | |||||||
|---|---|---|---|---|---|---|---|
| Cryptocrystalline | |||||||
| Amorphous | |||||||
| Miscellaneous | |||||||
| Notable varieties |
| ||||||
Titanium minerals | |||||
|---|---|---|---|---|---|
| Oxide minerals |
| ||||
| Silicate minerals | |||||
| Other | |||||
Gemmological classifications by E. Ya. Kievlenko (1980), updated | |||||||||
| Jewelry stones |
| ||||||||
| Jewelry-Industrial stones |
| ||||||||
| Industrial stones |
| ||||||||
Mineral identification | |
|---|---|
| "Special cases" ("native elements and organic minerals") |
|
|---|---|
| "Sulfides and oxides" |
|
| "Evaporites and similars" |
|
| "Mineral structures with tetrahedral units" (sulfate anion, phosphate anion, silicon, etc.) |
|
Associated Wikimedia
The following Wikimedia Foundation sister projects provide more on this subject:
-
 Commons
Commons
Free media repository -
 Wikibooks
Wikibooks
Free textbooks and manuals -
 Wikidata
Wikidata
Free knowledge base -
 Wikinews
Wikinews
Free-content news -
 Wikiquote
Wikiquote
Collection of quotations -
 Wikisource
Wikisource
Free-content library -
 Wikiversity
Wikiversity
Free learning tools -
 Wiktionary
Wiktionary
Dictionary and thesaurus
References
-
 List of all portalsList of all portals
List of all portalsList of all portals -
 The arts portal
The arts portal -
 Biography portal
Biography portal -
 Current events portal
Current events portal -
 Geography portal
Geography portal -
 History portal
History portal -
 Mathematics portal
Mathematics portal -
 Science portal
Science portal -
 Society portal
Society portal -
 Technology portal
Technology portal -
 Random portalRandom portal
Random portalRandom portal -
 WikiProject PortalsWikiProject Portals
WikiProject PortalsWikiProject Portals
