Layered materials
In material science, layered materials are solids with highly anisotropic bonding, in which two-dimensional sheets are internally strongly bonded, but only weakly bonded to adjacent layers.[1] Owing to their distinctive structures, layered materials are often suitable for intercalation reactions.[2][3]
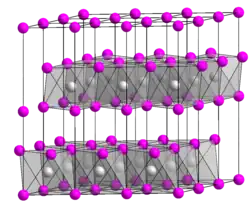
One large family of layered materials are metal dichalcogenides. In such materials, the M-chalcogen bonding is strong and covalent. These materials exhibit anisotropic electronic properties such as thermal and electrical conductivity.
Exfoliation
Because the layers bond to each other by relatively weak van der Waals forces, some layered materials are amenable to exfoliation, the complete separation of the layers of the material. Exfoliation can be done using sonication, mechanical, hydrothermal, electrochemical, laser-assisted, and microwave-assisted methods.[4]
Typically aggressive conditions are required involving highly polar solvents and reagents.[5] In the ideal case, exfoliation affords single-layer materials, such as graphene.
Examples
- metal dichalcogenides such as tantalum disulfide,[6] titanium disulfide,[7] and vanadium disulfide.
- graphite
- iron oxychloride
- layered double hydroxides, ionic solids where intercalated anions are exchangeable.[8]
References
- Lévy, Francis, ed. (1979). Intercalated Layered Materials. ISBN 978-94-009-9415-7.
- Kikkawa, S.; Kanamaru, F.; Koizumi, M. (1995). "Layered Intercalation Compounds". Inorganic Syntheses. Inorganic Syntheses. pp. 181–184. doi:10.1002/9780470132616.ch35. ISBN 9780470132616.
- Murphy, D. W.; Zahurak, S. M. (1995). "Lithium Insertion Compounds". Inorganic Syntheses. Inorganic Syntheses. pp. 185–191. doi:10.1002/9780470132616.ch36. ISBN 9780470132616.
- Zheng, Weiran; Lee, Lawrence Yoon Suk (2022). "Beyond sonication: Advanced exfoliation methods for scalable production of 2D materials". Matter. 5 (2): 515–545. doi:10.1016/j.matt.2021.12.010. S2CID 245902407.
- Nicolosi, V.; et al. (2013). "Liquid Exfoliation of Layered Materials". Science. 340 (6139). doi:10.1126/science.1226419. hdl:2262/69769. S2CID 177513486.
- Revelli, J. F.; Disalvo, F. J. (1995). "Tantalum Disulfide (TaS2 ) and Its Intercalation Compounds". Tantalum Disulfide (TaS2) and Its Intercalation Compounds. Inorganic Syntheses. Vol. 30. pp. 155–169. doi:10.1002/9780470132616.ch32. ISBN 9780470132616.
- Mckelvy, M. J.; Claunsinger, W. S. (1995). "Titanium Disulfide". Inorganic Syntheses. Inorganic Syntheses. Vol. 30. pp. 28–32. doi:10.1002/9780470132616.ch7. ISBN 9780470132616.
- Khan, Aamir I.; O'Hare, Dermot "Intercalation chemistry of layered double hydroxides: recent developments and applications" Journal of Materials Chemistry (2002), 12(11), 3191-3198. doi:10.1039/b204076j