CUMYL-CBMICA
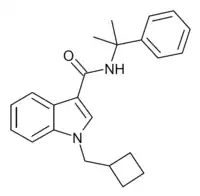
CUMYL-CBMICA (SGT-280) is an indole-3-carboxamide based synthetic cannabinoid receptor agonist which has been sold as a designer drug,[1][2] first being identified in Germany in August 2019. Since the structure fell outside the German drug analogue law provisions at the time, an amendment was made to the law to expand the relevant definition, which came into effect in April 2020.[3] It has been shown to act as a CB1 receptor agonist with an EC50 of 62.9nM.[4]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C23H26N2O |
| Molar mass | 346.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Halter S, Pulver B, Wilde M, Haschimi B, Westphal F, Riedel J, et al. (October 2020). "Cumyl-CBMICA: A new synthetic cannabinoid receptor agonist containing a cyclobutyl methyl side chain". Drug Testing and Analysis. 13 (1): 208–216. doi:10.1002/dta.2942. PMID 33037749.
- Polettini AE, Kutzler J, Sauer C, Bleicher S, Schultis W (September 2020). "LC-QToFMS Presumptive Identification of Synthetic Cannabinoids without Reference Chromatographic Retention/Mass Spectral Information. I. Reversed-Phase Retention Time QSPR Prediction as an Aid to Identification of New/Unknown Compounds". Journal of Analytical Toxicology. 45 (5): 429–439. doi:10.1093/jat/bkaa126. ISSN 0146-4760. PMID 32896861.
- "Draft Ordinance amending the Annex to the New Psychoactive Substances Act. Notification Number: 2020/173/D". Germany. 30 March 2020.
- Cannaert A, Sparkes E, Pike E, Luo JL, Fang A, Kevin RC, et al. (November 2020). "in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA". ACS Chemical Neuroscience. 11 (24): 4434–4446. doi:10.1021/acschemneuro.0c00644. PMID 33253529. S2CID 227246346.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.