Bromine nitrate
Bromine mononitrate is an inorganic compound, derived from bromine and nitric acid with the chemical formula BrNO3. The compound is a yellow liquid, decomposes at temperatures above 0 °C.[1]
 | |
| Names | |
|---|---|
| Other names
Bromine mononitrate, bromo nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BrNO3 | |
| Molar mass | 141.91 g/mol |
| Appearance | Yellow liquid |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 0 °C (32 °F; 273 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
1. Reaction of silver nitrate on an alcoholic solution of bromine:
- Br2 + AgNO3 → BrNO3 + AgBr
2. Reaction of bromine chloride with chlorine nitrate at low temperatures:
- BrCl + ClNO3 → BrNO3 + Cl2
Physical properties
Bromine mononitrate forms an unstable yellow liquid that decomposes at temperatures above 0 °C.
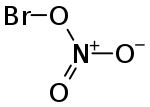
The molecule has the structure BrONO2.[2][3]
The compound is easily soluble in trichlorofluoromethane and carbon tetrachloride.
Applications
Bromine nitrate plays a role in tropospheric chemistry as it reacts with sulfuric acid.[4][5]
References
- "Bromine nitrate properties - SpringerMaterials". materials.springer.com. Retrieved 31 October 2021.
- Colussi, Agustín J.; Grela, María A. (1998). "Thermochemical kinetics of bromine nitrate, bromine nitrite, halogen hydroperoxides, dichlorine pentoxide, peroxycarboxylic acids, and diacyl peroxides". International Journal of Chemical Kinetics. 30 (1): 41–45. doi:10.1002/(SICI)1097-4601(1998)30:1<41::AID-KIN5>3.0.CO;2-U. ISSN 1097-4601. Retrieved 31 October 2021.
- Parthiban, Srinivasan; Lee, Timothy J. (8 July 1998). "Ab initio investigation of the atmospheric molecule bromine nitrate: Equilibrium structure, vibrational spectrum, and heat of formation". The Journal of Chemical Physics. 109 (2): 525–530. doi:10.1063/1.476589. ISSN 0021-9606. Retrieved 31 October 2021.
- Sander, R.; Rudich, Y.; Glasow, R. von; Crutzen, P. J. (1999). "The role of BrNO3 in marine tropospheric chemistry: A model study". Geophysical Research Letters. 26 (18): 2857–2860. doi:10.1029/1999GL900478. ISSN 1944-8007. S2CID 94609017. Retrieved 31 October 2021.
- Spencer, John E.; Rowland, F. S. (1 January 1978). "Bromine nitrate and its stratospheric significance". The Journal of Physical Chemistry. 82 (1): 7–10. doi:10.1021/j100490a002. ISSN 0022-3654. Retrieved 31 October 2021.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.