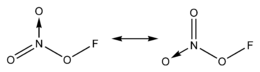
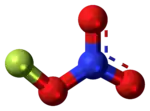
Fluorine nitrate
Fluorine nitrate is an unstable derivative of nitric acid with the formula FNO
3. It is shock-sensitive.[1] Due to its instability, it is often produced from chlorine nitrate as needed.
 | |
 | |
| Names | |
|---|---|
| Other names
Nitryl hypofluorite | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| FNO3 | |
| Molar mass | 81.002 g·mol−1 |
| Density | 2.217 g/L[1] |
| Melting point | −175 °C (−283.0 °F; 98.1 K) |
| Boiling point | −46 °C (−51 °F; 227 K) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
+10.46 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Explosive gas |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and properties
Fluorine nitrate is formed when fluorine gas is bubbled through nitric acid or reacted with solid potassium nitrate:[2]
- F2 + HNO3 → FNO3 + HF
- F2 + KNO3 → FNO3 + KF
It decomposes in water to form oxygen gas, oxygen difluoride, hydrofluoric acid, and nitric acid.[1]
In fluorine nitrate, the oxygen atom bridging nitrogen and fluorine is in a rare oxidation state of 0 due to its electronegativity being lower than that of fluorine but higher than that of nitrogen.
References
- Ruff, Otto; Kwasnik, Walter (1935). "The fluorination of nitric acid. The nitroxyfluoride, NO3F". Angewandte Chemie. 48: 238–240. doi:10.1002/ange.19350481604.
- Yost, Don M.; Beerbower, Alan. "The Reaction of Fluorine with Nitric acid and with Solid Potassium Nitrate to Form NO3F". Communication.
{{cite journal}}: Cite journal requires|journal=(help)
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.