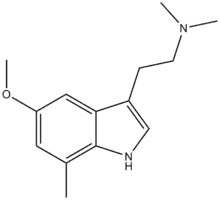
5-Methoxy-7,N,N-trimethyltryptamine
5-Methoxy-7,N,N-trimethyltryptamine (5-MeO-7,N,N-TMT, 5-MeO-7-TMT), is a tryptamine derivative which acts as a partial agonist at the 5-HT2 serotonin receptors, with an EC50 of 63.9 nM and an efficacy of 66.2% at 5-HT2A (vs 5-HT), and weaker activity at 5-HT2B and 5-HT2C.[1][2][3] In animal tests, both 7,N,N-TMT and 5-MeO-7,N,N-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT and 5-MeO-DMT, but compounds with larger 7-position substituents such as 7-ethyl-DMT and 7-bromo-DMT did not produce psychedelic-appropriate responding despite high 5-HT2 receptor binding affinity, suggesting these may be antagonists or weak partial agonists for the 5-HT2 receptors.[4] The related compound 7-MeO-MiPT (cf. 5-MeO-MiPT) was also found to be inactive, suggesting that the 7-position has poor tolerance for bulky groups at this position, at least if agonist activity is desired.[5]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H20N2O |
| Molar mass | 232.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
References
- Banister S, Jorgensen W, Jinlong T. Compounds. Patent WO 2023/115166
- Benington F, Morin RD, Bradley RJ (1976). "7-(N,N-Trimethyl)-5-methoxytryptamine". Journal of Heterocyclic Chemistry. 13 (4): 749–751. doi:10.1002/jhet.5570130412.
- Lyon RA, Titeler M, Seggel MR, Glennon RA (January 1988). "Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens". European Journal of Pharmacology. 145 (3): 291–7. doi:10.1016/0014-2999(88)90432-3. PMID 3350047.
- Glennon RA, Schubert E, Jacyno JM, Rosecrans JA (November 1980). "Studies on several 7-substituted N,N-dimethyltryptamines". Journal of Medicinal Chemistry. 23 (11): 1222–6. doi:10.1021/jm00185a014. PMID 6779006.
- Repke DB, Grotjahn DB, Shulgin AT (July 1985). "Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents". Journal of Medicinal Chemistry. 28 (7): 892–6. doi:10.1021/jm00145a007. PMID 4009612.