Lithium tungstate
Lithium tungstate is the inorganic compound with the formula Li2WO4. It is a white solid that is soluble in water. The compound is one of the several orthotungstates, compounds that feature the tetrahedral WO42− anion.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.602 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| Li2WO4 | |
| Appearance | white solid |
| Density | 4.56 g/cm3 |
| Related compounds | |
Other cations |
Sodium tungstate Caesium tungstate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
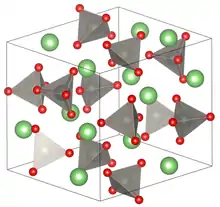
The salt consists of tetrahedrally coordinated Li and W centres bridged by oxides. The W-O and Li-O bond distances are 1.79 and 1.96 Å, respectively. These differing bond lengths reflect the multiple bond character of the W-O interaction and the weaker ionic bonding between the Li-O interactions.[1] The solid undergoes phase transitions at high pressures, such that the coordination geometry at tungsten becomes octahedral (six W-O bonds). For example at 40 kilobars, it adopts a structure related to wolframite.[2]
Uses
Lithium tungstate is used to produce high density aqueous polytungstate (metatungstate) solutions. Like other high density fluids, such solutions are often used in the separation of minerals and other solids. These can achieve a density of 2.95 at 25 °C and up to 3.6 in hot water.[3]
This use of lithium and sodium tungstate anions was developed in the 1980s and early 1990s to address toxicity and safety issues with the existing organic high density fluids. Unlike methylene iodide and bromoform, polytungstates and heteropolytungstates can be more safely be used in an indoor environment without a fume hood with only ordinary common sense safety precautions such as protective gloves and safety glasses.[4]
References
- Zachariasen, W. H; Plettinger, H. A. "The crystal structure of lithium tungstate" Acta Crystallographica 1961, volume 14, pp. 229-30. doi:10.1107/S0365110X61000772
- Horiuchi, Hiroyuki Morimoto, Nobuo; Yamaoka, Shinobu "The crystal structure of lithium tungstate phase (IV) and its relation to the wolframite-type structure" Journal of Solid State Chemistry volume 33, 115-19. doi:10.1016/0022-4596(80)90554-X
- Manufacturer websites , "LST Heavy Liquid". Archived from the original on 2015-02-26. Retrieved 2014-10-12. and
- MSDS and safety information "Archived copy" (PDF). Archived from the original (PDF) on 2013-10-19. Retrieved 2013-04-26.
{{cite web}}: CS1 maint: archived copy as title (link) "Health and safety". Archived from the original on 2015-02-26. Retrieved 2014-10-12.