Branaplam
Branaplam (development codes LMI070 and NVS-SM1) is a pyridazine derivative that is being studied as an experimental drug. It was originally developed by Novartis to treat spinal muscular atrophy (SMA); since 2020 it was being developed to treat Huntington's disease (HD) but the trial ended in 2023 with harmful side effects.[1]
 | |
| Clinical data | |
|---|---|
| Other names | LMI070; NVS-SM1 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
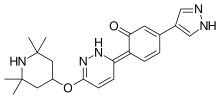
| Formula | C22H27N5O2 |
| Molar mass | 393.491 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
As a treatment for SMA, branaplam increases the amount of functional survival of motor neuron protein produced by the SMN2 gene through modifying its splicing pattern.[2][3] It was studied since 2014 in a clinical trial in children with SMA type 1[4][5][6] until the programme was discontinued in 2021.
In October 2020, Novartis announced that branaplam lowers the level of huntingtin protein, which is one of the major therapeutic approaches in Huntington's disease. In 2021, U.S. Food and Drug Administration (FDA) granted an orphan drug status to branaplam for treatment of Huntington’s disease, and Novartis announced that they will set up clinical trials in 2021.[7] In December 2021, branaplam received a Fast Track designation from the FDA towards a phase IIb study in adult patients with early-stage HD manifestation.[8][9]
References
- https://www.fiercebiotech.com/biotech/novartis-cans-branaplam-after-seeing-huntingtons-safety-signal-delays-orphan-drug-over-slow
- Palacino J, Swalley SE, Song C, Cheung AK, Shu L, Zhang X, et al. (July 2015). "SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice". Nature Chemical Biology. 11 (7): 511–7. doi:10.1038/nchembio.1837. PMID 26030728.
- "LMI070". SMA News Today. Retrieved 2017-03-10.
- "An Open Label Study of LMI070 in Type 1 Spinal Muscular Atrophy (SMA)". ClinicalTrials.gov. Retrieved 2017-03-10.
- "Novartis Releases Update on LMI070 (Branaplam) Clinical Trial". CureSMA. 2017-09-20. Archived from the original on 2017-11-25. Retrieved 2017-10-07.
- "| Novartis announced that enrollment for the ongoing clinical trial of branaplan is now closed". 20 May 2019. Retrieved 2019-07-12.
- "Novartis receives US Food and Drug Administration (FDA) Orphan Drug Designation for branaplam (LMI070) in Huntington's disease (HD)". novartis.com. Retrieved 2020-10-24.
- MS, Marisa Wexler. "Branaplam for Huntington's on FDA's Fast Track; Phase 2 Trial Enrolling".
- "Novartis receives FDA Fast Track designation for branaplam (LMI070) for the treatment of Huntington's Disease". Novartis.