Aluminium cyanide
Aluminium cyanide is a metallic cyanide with a chemical formula of Al(CN)3.[1] It is a white solid that undergoes hydrolysis to produce aluminium hydroxide and hydrogen cyanide.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C3AlN3 | |
| Molar mass | 105.036 g·mol−1 |
| Appearance | white solid |
| Reacts | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and properties
Aluminium cyanide was first produced in 1924 as its ammoniate, Al(CN)3·5NH3, by reacting aluminium metal and mercury(II) cyanide in liquid ammonia to prevent hydrolysis.[1]
- 2 Al + 3 Hg(CN)2 → 2 Al(CN)3 + 3 Hg
When the ammoniate contacts water, it produces aluminium hydroxide, ammonia, and ammonium cyanide.[1]
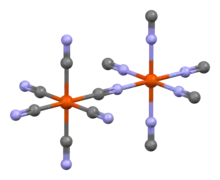
The pure compound was produced in 2001 by the reaction of lithium tetrachloroaluminate and trimethylsilyl cyanide in diethyl ether and its crystals form an octahedral Prussian-blue-type structure.[3]
References
- Bergstrom, F. W. (July 1924). "The Reaction Between Mercuric Cyanide and Certain Metals in Liquid Ammonia". Journal of the American Chemical Society. 46 (7): 1559–1568. doi:10.1021/ja01672a002.
- Axel Schulz; Jonas Surkau (2022). "Main group cyanides: from hydrogen cyanide to cyanido-complexes". Reviews in Inorganic Chemistry. 43 (1): 49–188. doi:10.1515/revic-2021-0044.
- Darrick Williams; Brett Pleune; Kurt Leinenweber; J. Kouvetakis (2001). "Synthesis and Structural Properties of the Binary Framework C–N Compounds of Be, Mg, Al, and Tl". Journal of Solid State Chemistry. 159 (1): 244–250. doi:10.1006/jssc.2001.9192.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.