Rodents: Somatosensory Perception of Whiskers
Introduction
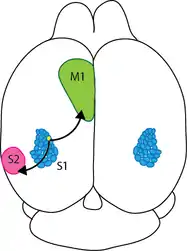

The barrel Cortex is a specialized region in somatosensory cortex responsible for processing the tactile information from whiskers. As every other cortical region, the barrel cortex also preserves the columnar organization which plays a crucial role in information processing. Information from each whisker is represented in separate, discrete columns analogous to “barrels”, hence the name barrel cortex. Rodents use whiskers constantly to acquire sensory information from the environment. Given their nocturnal nature, tactile information carried by whisker forms the primary sensory signals to build a perceptual map of the environment. The whiskers on the snouts of mice and rats serve as arrays of highly sensitive detectors for acquiring tactile information as shown in Figure 1 A and B. By using their whiskers, rodents can build spatial representations of their environment, locate objects, and perform fine-grain texture discrimination. Somatosensory whisker-related processing is highly organized into stereotypical maps, which occupy a large portion of the rodent brain. During exploration and palpation of objects, the whiskers are under motor control, often executing rapid large-amplitude rhythmic sweeping movements, and this sensory system is therefore an attractive model for investigating active sensory processing and sensory-motor integration. In these animals, a large part of the neocortex is dedicated to the processing of information from the whiskers. Since rodents are nocturnal, visual information is relatively poor and they rely heavily on the tactile information from whiskers. Perhaps the most remarkable specialization of this sensory system is the primary somatosensory ‘‘barrel’’ cortex, where each whisker is represented by a discrete and well-defined structure in layer 4.
These layer 4 barrels are somatotopically arranged in an almost identical fashion to the layout of the whiskers on the snout i.e. bordering whiskers are represented in adjacent cortical areas [1]. Sensorimotor integration of whisker related activity leads to pattern discrimination and enables rodents to have a reliable map of the environment. This is an interesting model to study because rodents use whisker to “see” and this cross modality sensory information processing could help us to improve the life of humans, who are deprived of one sensory modality. Specifically, blind people can be trained to use somatosensory information to build a spatial map of the environment [2].
Pathways carrying whisker information to Barrel Cortex
|
Pathways carrying whisker information to Barrel Cortex
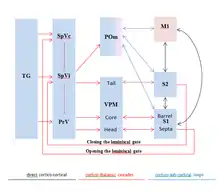 Figure 2. Schematic demonstrating the ascending pathway of rodent whisker-related sensorimotor system.
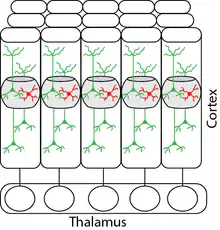 Figure 3.Processing of whisker-related sensory information in barrel cortex. System level description of the pathways involved in the propagation of information from whiskers to cortex & columnar organization of the barrel cortex which receives information from single whisker.
The sensory neurons make excitatory glutamatergic synapses in the trigeminal nuclei of the brain stem. Trigemino-thalamic neurons in the principal trigeminal nucleus are organized into somatotopically arranged ‘‘barrelettes,’’ each receiving strong input from a single whisker as shown in (Figure 3). The principal trigeminal neurons project to the ventral posterior medial (VPM) nucleus of the thalamus, which is also somatotopically laid out into anatomical units termed ‘‘barreloids’’ VPM neurons respond rapidly and precisely to whisker deflection, with one ‘‘principal’’ whisker evoking stronger responses than all others. The axons of VPM neurons within individual barreloids project to the primary somatosensory neocortex forming discrete clusters in layer 4, which form the basis of the ‘‘barrel’’ map as shown in Figure 3.
|
Whisker information processing in Barrel Cortex with specialized local microcircuit
The deflection of a whisker is thought to open mechano-gated ion channels in nerve endings of sensory neurons innervating the hair follicle (although the molecular signalling machinery remains to be identified). The resulting depolarization evokes action potential firing in the sensory neurons of the infraorbital branch of the trigeminal nerve. The transduction through mechanical deformation is similar to the hair cells in the inner ear; in this case the contact of whiskers with the objects causes the mechano-gated ion channels to open. Cation-permeable ion channels let positively charged ions into the cells and causes depolarization, eventually leading to generation of action potentials. A single sensory neuron only fires action potentials to deflection of one specific whisker. The innervation of the hair follicle shows a diversity of nerve endings, which may be specialized for detecting different types of sensory input [3].
The layer 4 barrel map is arranged almost identically to the layout of the whiskers on the snout of the rodent. There are several recurrent connections in layer 4 and it sends axons to layer 2/3 neurons, which integrates information from other cortical regions like primary motor cortex. These intra-cortical and inter-cortical connections enable the rodents to achieve stimulus discrimination capabilities and to extract optimal information from the incoming tactile stimulus. Also, these projections play a crucial role in integrating somatosensory information with motor output. Information from whiskers is processed in the barrel cortex with specialized local microcircuits formed to extract optimal information about the environment. These cortical microcircuits are composed of excitatory and inhibitory neurons as shown in Figure 4.

Learning whisker based object discrimination & texture differentiation
Rodents move their sensors to collect information, and these movements are guided by sensory input. When action sequences are required to achieve success in novel tasks, interactions between movement and sensation underlie motor control [4] and complex learned behaviours [5]. The motor cortex has important roles in learning motor skills [6-9], but its function in learning sensorimotor associations is unknown. The neural circuits underlying sensorimotor integration are beginning to be mapped. Different motor cortex layers harbour excitatory neurons with distinct inputs and projections [10-12]. Outputs to motor centres in the brain stem and spinal cord arise from pyramidal tract-type neurons in layer 5B (L5B). Within motor cortex, excitation descends from L2/3 to L5 [13, 14]. Input from somatosensory cortex impinges preferentially onto L2/3 neurons. L2/3 neurons [10] therefore directly link somatosensation and control of movements. In one of the recent studies [15], mice were trained head fixed in a vibrissa-based object-detection task while imaging populations of neurons [16]. Following a sound, a pole was moved to one of several target positions within reach of the whiskers (the ‘go’ stimulus) or to an out-of-reach position (the ‘no-go’ stimulus). Target and out-of-reach locations were arranged along the anterior–posterior axis; the out-of reach position was most anterior. Mice searched for the pole with one whisker row, the C row, and reported the pole as ‘present’ by licking, or ‘not present’ by withholding licking. Licking on go trials (hit) was rewarded with water, whereas licking on no-go trials (false alarm) was punished with a time-out during which the trial was stopped for 2 seconds. Trials without licking (no-go, correct rejection, go, and miss) were not rewarded or punished. All mice showed learning within the first two or three sessions. Performance reached expert levels after three to six training sessions. Learning the behavioural task was directly dependent on the motor related behaviour. Naive mice whisked occasionally in a manner unrelated to trail structure. Thus, object detection relies on a sequence of actions, linked by sensory cues. An auditory cue triggers whisking during the sampling period. Contact between whisker and object causes licking for a water reward during a response period. Silencing vM1 indicates that this task requires the motor cortex; with vM1 silenced, task-dependent whisking persisted, but was reduced in amplitude and repeatability, and task performance dropped.
Neural Correlates of Sensorimotor learning mechanism
Coding of touch in the motor cortex is consistent with direct input from vS1 to the imaged neurons. A model based on population coding of individual behavioural features also predicted motor behaviours. Accurate decoding of whisking amplitude, whisking set-point and lick rate suggests that vM1 controls these slowly varying motor parameters, as expected from previous motor cortex and neurophysiological experiments.
References
1 Feldmeyer D, Brecht M, Helmchen F, Petersen CCH, Poulet JFA, Staiger JF, Luhmann HJ, Schwarz C."Barrel cortex function" Progress in Neurobiology 2013, 103 : 3-27.
2 Lahav O, Mioduser D. "Multisensory virtual environment for supporting blind persons' acquisition of spatial cognitive mapping, orientation, and mobility skills." Academia.edu 2002.
3 Alloway KD. "Information processing streams in rodent barrel cortex: The differential functions of barrel and septal circuits." Cereb Cortex 2008, 18(5):979-989.
4 Scott SH. "Inconvenient truths about neural processing in primary motor cortex." The Journal of physiology 2008, 586(5):1217-1224.
5 Wolpert DM, Diedrichsen J, Flanagan JR. "Principles of sensorimotor learning." Nature reviews Neuroscience 2011, 12(12):739-751.
6 Wise SP, Moody SL, Blomstrom KJ, Mitz AR. "Changes in motor cortical activity during visuomotor adaptation." Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale 1998, 121(3):285-299.
7 Rokni U, Richardson AG, Bizzi E, Seung HS. "Motor learning with unstable neural representations." Neuron 2007, 54(4):653-666.
8 Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. "Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice." Nature 2010, 464(7292):1182-1186.
9 Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. "Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning." The Journal of neuroscience : the official journal of the Society for Neuroscience 2011, 31(7):2481-2487.
10 Keller A. "Intrinsic synaptic organization of the motor cortex." Cereb Cortex 1993, 3(5):430-441.
11 Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. "Long-range neuronal circuits underlying the interaction between sensory and motor cortex." Neuron 2011, 72(1):111-123.
12 Hooks BM, Hires SA, Zhang YX, Huber D, Petreanu L, Svoboda K, Shepherd GM. "Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas." PLoS biology 2011, 9(1):e1000572.
13 Anderson CT, Sheets PL, Kiritani T, Shepherd GM. "Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex." Nature neuroscience 2010, 13(6):739-744.
14 Kaneko T, Cho R, Li Y, Nomura S, Mizuno N. "Predominant information transfer from layer III pyramidal neurons to corticospinal neurons." The Journal of comparative neurology 2000, 423(1):52-65.
15 O'Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. "Vibrissa-based object localization in head-fixed mice." The Journal of neuroscience : the official journal of the Society for Neuroscience 2010, 30(5):1947-1967.
16 O'Connor DH, Peron SP, Huber D, Svoboda K. "Neural activity in barrel cortex underlying vibrissa-based object localization in mice." Neuron 2010, 67(6):1048-1061.
17 Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. "Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein." Nature biotechnology 2004, 22(12):1567-1572.
18 Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER. "Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators." Nature methods 2009, 6(12):875-881.
Snakes: Sensing of Infrared Radiation
Introduction
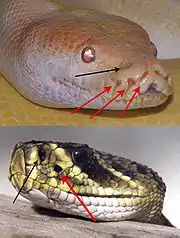
When seeing or sometimes even thinking of snakes, many people feel uncomfortable or even scared. There is a reason that they are considered being mythical. Snakes are different when compared to other animals: they do not have legs, they are long and move elegantly and without a noise, some of them are venomous and they steadily use their forked tongue to smell. Some of them are fast and effective killers even by night. Something that definitely makes them special is their „sixth sense“: the ability to detect infrared radiation. Similar to night viewers, snakes are capable of detecting heat changes in their surroundings and thus obtaining a detailed picture of it. There are at least two different groups of snakes which have separately developed this ability: in the first are the pit vipers, and in the second boas and pythons (those two are often classified into one group called “boids”). However, snakes are not the only species which have evolved this sense: vampire bats and some groups of insects have also developed it. Even at night pit vipers, boas and pythons can make out rodents due to the heat they emit. It can be detected by a sensory system that allows them to „see“ electromagnetic radiation with long wavelengths ranging from 750 nm to 1 mm. The organs which make that possible are called “pit organs”, and are located under their eyes, inside two hollows of the maxilla bone. They are immensely sensitive as they can even detect changes in temperature of as little as 0.003K.
Anatomy of Sensing Organ

The infrared-sensing organs of vipers and boids are similar in their physiological structure but differ in their number, location and morphology. The anatomy is quite simple and will be explained in the example of the crotalus, a venomous pit viper found only in the Americas from southern Canada to northern Argentina. It consists of a hollow space that is separated into two air-filled chambers by a thin membrane of the thickness of 0.01 mm. It is filled with sensory cells of the trigenimal nerve (TNM). Roughly 7000 in number, they transduce the heat through heat sensitive ion channels, and increase their firing rate when a positive change in temperature occurs and decrease in the opposite case. They are very sensitive due to the spatial proximity of these thermoreceptors to the outside and also because of the air-filled chamber that lies underneath. This air-filled chamber works as an insulator in separating tissues that would otherwise quickly exchange heat energy. Thus, the absorbed thermal energy is used exclusively by the sensory system and is not lost to lower-lying tissues. This simple but sophisticated anatomy is the reason for the unique sensitivity of the pit organs. The pit organs’s physique allows even the detection of the radiation’s direction. The external opening is roughly half as large as the membrane. Thus, the whole organ works according to the optics of a pinhole camera: the position of the irradiated spot provides information about the object’s location. The heat itself is detected by the activation of heat sensitive ion channels called TRPA1. In other animals these channels also exist but have other functions like detecting chemical irritants or cold. Pit vipers and boids seem to have evolved the infrared-sensing independently. Since the heat sensitive ion channels have different thermal thresholds in different snakes, the temperature sensitivity differs among the snakes. Crotalus have the most sensitive channels. Snakes that are not able to detect infrared radiation also possess those channels, but their thermal threshold is too high to detect infrared radiation.
Brain’s Anatomy
Every sensory organ has a dedicated brain region to process the collected information. Snakes evaluate infrared sensory input from the pit organs in the nucleus of the lateral descending trigeminal tract (“LTTD”), a unique region in their metencephalon which has not been found in other animals. The LTTD is linked to the tectum opticum via the reticularis caloris (“RC”). Its function is still unknown. In the tectum opticum visual and infrared stimuli are connected, in order to provide a detailed idea of the animal’s surrounding.
Physiology
Experiments have shown that the detection of heat targets must be quite accurate as snakes hit thermal sources with a low error even without the help of vision. Measurements have determined that the opening angle of an infrared beam falling onto the pit organ is 45 to 60 degrees. Depending on where the heat source is relatively to the snake, the beam hits the pit’s membrane on a different spot. The receptive field of the infrared sensing system on the tectum opticum is similarly represented as the visual receptive field. The front-end of the tectum opticum receives its input from the back part of the pit membrane and the retina, and thus processes stimuli from the front part of the visual field. Similarly, the back and the sides of the visual field are represented in the back part of the tectum opticum and the front part of the pit membrane and the retina. The receptive fields of the visual and infrared sensory systems overlap almost perfectly within the tectum opticum, such that the neurons there receive and process sensory information from two senses, from more or less the same direction. While crotalus only have two pit organs, the anatomy of the temperature sensors is much more complicated in boas and pythons. They possess 13 pit organs on each side of the head. Every one of those also works like a pinhole camera that reverses the picture. The information of the front part of the visual field is again processed in the front part of the tectum opticum but now, the receptive field of every pit organ is projected onto a different part of it. The front pit organs are represented in the front part of the tectum opticum and the back parts in the back. In addition, the receptive fields of the different pit organs overlap, and thus provide a more or less continuous projective field that matches the visual one. It is curious that the front part of every pit organ is projected to the back part of the receptive field in the tectum opticum, an organization that is quite complicated and unique. The tectum opticum contains six different kinds of neurons which fire for infrared and/or visual stimuli. Some cell types respond only if there is a visual and an infrared stimulus, while others respond for any kind of stimulus. There are cells that respond for one of the sensory input if it comes alone, but increases its firing rate for simultaneous input from both systems. The last group of cells works the other way around. Some of them respond strongly for visual stimuli and stop firing when stimuli from the pit organs also arrive or vice versa. What do snakes with pit organs need these different kinds of neurons for? The processing in their brain has to help the snakes with different tasks: first of all, the snake should be able to detect and locate stimuli. Second, they have to be identified and reacted to appropriately. The cells that respond to both visual and infrared stimuli independently from each other could be responsible for the first task. Cells that only respond if they get both stimuli at the same time could work as detectors for living, moving objects. Moreover, cells that stop firing as soon as the visual stimuli is completed with an infrared signal could be especially important for detecting the cool surrounding like leaves or trees. The interaction between the different types of cells are important for correctly identifying the stimuli. They are not only used for identifying warm-blooded prey, but also for identifying predators and the snake’s thermoregulation.

Infrared Sensing in Vampire Bats
Vampire bats are the only mammals that are able to detect infrared radiation. To do so they have three hollows in their nose which contain the sensing organs. While they also use ion channels to detect heat, it is a different type of ion channels than in snakes. In other mammals and even everywhere in their own body except for the nose this type of molecule is responsible for sensing pain and burning. However, in the nose the threshold is much lower. The channel already detects changes in temperature from 29 °C on. This allows vampire bats to locate heat sources at a distance of 20 cm and helps them to find blood-rich spots on their prey.
References
Newman, E.A., and Hartline, P.H. (1982) Infrared "vision" in snakes. Scientific American 246(3):116-127 (March).
Gracheva et al.: Molecular Basis of Infrared Detection by Snakes. Nature. 2010 April 15; 464(7291): 1006-1011
Campbell et. Al.: Biological infrared Imaging and sensing. Micron 33 (2002) 211-225
Gracheva et al.: Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature.476, 88-91 (04.08.2011)