Uranium trifluoride
Uranium trifluoride is an inorganic chemical compound with the chemical formula UF3.
 | |
| Names | |
|---|---|
| Other names
Trifluorouranium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.990 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| UF3 | |
| Molar mass | 295.024 g/mol[1] |
| Appearance | purple solid[2] |
| Density | 8.9 g/cm3[1] |
| Melting point | 1,495 °C (2,723 °F; 1,768 K)[1] |
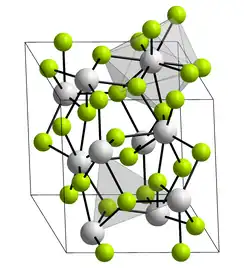
| Structure | |
| Rhombohedral, hP24 | |
| P63cm, No. 185[3] | |
a = 0.7181 nm, c = 0.7348 nm | |
Lattice volume (V) |
0.32815 |
Formula units (Z) |
6 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Uranium trifluoride can be obtained by reacting uranium(IV) fluoride with aluminium at 900 °C or with uranium:[4]
References
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.97. ISBN 1-4398-5511-0.
- Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2007). Lehrbuch der anorganischen Chemie (in German). Berlin. p. 1969. ISBN 978-3-11-017770-1. OCLC 180963521.
{{cite book}}: CS1 maint: location missing publisher (link) - Zachariasen, W. H. (1949). "Crystal chemical studies of the 5f-series of elements. XII. New compounds representing known structure types" (PDF). Acta Crystallographica. 2 (6): 388–390. doi:10.1107/S0365110X49001016.
- Brauer, Georg (ed.) (1978) Handbuch der Präparativen Anorganischen Chemie. Vol 3. Enke, Stuttgart. ISBN 3-432-87813-3. p. 1207
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.