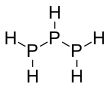
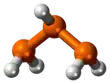
Triphosphane
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Triphosphane[1] | |||
| Other names
Triphosphine[2] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| P3H5 | |||
| Molar mass | 97.96099 g·mol−1 | ||
| Appearance | Colourless gas | ||
| Related compounds | |||
Other anions |
triazane | ||
Related Binary phosphanes |
phosphane diphosphane | ||
Related compounds |
triazene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Triphosphane (IUPAC systematic name) or triphosphine is an inorganic compound having the chemical formula HP(PH2)2. It can be generated from diphosphine but is highly unstable at room temperature:[3]
- 2 P2H4 → P3H5 + PH3
Samples have been isolated by gas chromatography. The compound rapidly converts to PH3 and the cyclophosphine cyclo-P5H5.[4]
References
- "triphosphane (CHEBI:35893)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. 7 June 2006. Main. Retrieved 27 September 2011.
- "Triphosphine". NIST Chemistry WebBook. USA: National Institute of Standards and Technology. Retrieved 27 September 2011.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Marianne Baudler, Klaus Glinka (1993). "Monocyclic and Polycyclic Phosphines". Chem. Rev. 93: 1623–1667. doi:10.1021/cr00020a010.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.