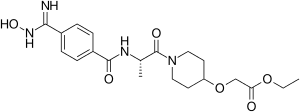
Sibrafiban
Sibrafiban (Ro 48–3657, proposed brand name Xubix) is the double prodrug of Ro-44-3888, which is a platelet aggregation inhibitor. It was being developed for secondary prevention of arterial thrombosis following unstable angina pectoris and acute myocardial infarction (MI).[1] On August 6, 1999, Hoffmann-La Roche announced that the preliminary results from Phase III clinical trials had not shown that sibrafiban was better than aspirin in preventing recurrent ischemic events in patients with acute coronary syndrome. The development of sibrafiban was terminated.
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H28N4O6 |
| Molar mass | 420.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Dooley M, Goa KL (February 1999). "Sibrafiban". Drugs. 57 (2): 225–30, discussion 231–2. doi:10.2165/00003495-199957020-00012. PMID 10188763.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.