sec-Butylamine
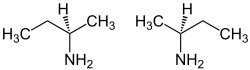 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butan-2-amine | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| Abbreviations | 2-AB |
| 1361345, 1718761 (R), 1718760 (S) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.288 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 2733 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.139 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 0.724 g cm−3 |
| Melting point | −104.50 °C; −156.10 °F; 168.65 K |
| Boiling point | 63 °C; 145 °F; 336 K |
| Miscible[1] | |
Refractive index (nD) |
1.3928 |
| Viscosity | 500 μPa s (at 20 °C) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−138.5 to −136.5 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−3.0095 to −3.0077 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
 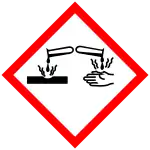  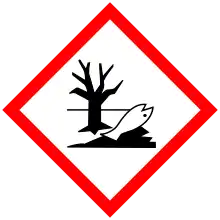 | |
| Danger | |
| H225, H302, H314, H332, H400 | |
| P210, P273, P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | 19 °C (66 °F; 292 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Related compounds | |
Related alkanamines |
|
Related compounds |
2-Methyl-2-nitrosopropane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
sec-Butylamine is an organic chemical compound (specifically, an amine) with the formula CH3CH2CH(NH2)CH3. It is a colorless liquid. sec-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, tert-butylamine, and isobutylamine. sec-Butylamine is chiral and therefore can exist in either of two enantiomeric forms.
sec-Butylamine is used in the production of some pesticides.[2]
References
- "ICSC 0401 - sec-BUTYLAMINE".
- Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- United States Environmental Protection Agency. "Bromacil". 1996, pp. 1–11. Accessed 9 October 2012
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.