Isobutylamine
Isobutylamine is an organic chemical compound (specifically, an amine) with the formula (CH3)2CHCH2NH2, and occurs as a colorless liquid.[1][2] Isobutylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and tert-butylamine. It is the decarboxylated form of the amino acid valine, and the product of the metabolism thereof by the enzyme valine decarboxylase.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpropan-1-amine | |
| Other names
(2-Methylpropyl)amine | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 385626 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.042 |
| EC Number |
|
| 81862 | |
| KEGG | |
| MeSH | isobutylamine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1214 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.139 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 736 mg mL−1 |
| Melting point | −86.6 °C; −124.0 °F; 186.5 K |
| Boiling point | 67 to 69 °C; 152 to 156 °F; 340 to 342 K |
| Miscible | |
| -59.8·10−6 cm3/mol | |
Refractive index (nD) |
1.397 |
| Viscosity | 500 μPa s (at 20 °C) |
| Thermochemistry | |
Heat capacity (C) |
194 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−133.0–−132.0 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−3.0139–−3.0131 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
 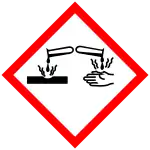  | |
| Danger | |
| H225, H301, H314 | |
| P210, P280, P301+P310, P305+P351+P338, P310 | |
| Flash point | −9 °C (16 °F; 264 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
224 mg kg−1 (oral, rat) |
| Related compounds | |
Related alkanamines |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Isobutylamine is an odorant binding to TAAR3 in mice and can trigger sexual behaviour in male mice dependent on the cluster of TAAR2 through TAAR9.[3]
References
- Isobutylamine chemicalbook.com
- Isobutylamine Chemblink.com
- Harmeier A, Meyer CA, Staempfli A, Casagrande F, Petrinovic MM, Zhang YP, Künnecke B, Iglesias A, Höner OP, Hoener MC (2018). "How Female Mice Attract Males: A Urinary Volatile Amine Activates a Trace Amine-Associated Receptor That Induces Male Sexual Interest". Frontiers in Pharmacology. 9: 924. doi:10.3389/fphar.2018.00924. PMC 6104183. PMID 30158871.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.