Rebamipide
Rebamipide, an amino acid derivative of 2-(1H)-quinolinone, is used for mucosal protection,[1] healing of gastroduodenal ulcers, and treatment of gastritis.[2] It works by enhancing mucosal defense, scavenging free radicals,[3] and temporarily activating genes encoding cyclooxygenase-2.[4]
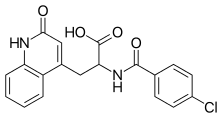 | |
| Clinical data | |
|---|---|
| Trade names | Mucosta (JP), Rebagen (KR, CN, IN), Rebagit (RU), Rebamax (ID) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H15ClN2O4 |
| Molar mass | 370.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Studies have shown that rebamipide can fight the damaging effects of NSAIDs on the GIT mucosa,[5] and more recently, the small intestine, but not for naproxen-induced gastric damage.[6]
Availability
Rebamipide is used in a number of Asian countries including Japan (marketed as Mucosta), South Korea, China[7] and India (where it is marketed under the trade name Rebagen). It is also approved in Russia under the brand name Rebagit.[8]
References
- Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A (September 1998). "Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing". Digestive Diseases and Sciences. 43 (9 Suppl): 5S–13S. PMID 9753220.
- Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K (November 1995). "Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine". Digestive Diseases and Sciences. 40 (11): 2469–72. doi:10.1007/bf02063257. PMID 7587834. S2CID 22807270.
- Takumida M, Anniko M (January 2009). "Radical scavengers for elderly patients with age-related hearing loss". Acta Oto-Laryngologica. 129 (1): 36–44. doi:10.1080/00016480802008215. PMID 18607930. S2CID 16906464.
- Tarnawski AS, Chai J, Pai R, Chiou SK (February 2004). "Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action?". Digestive Diseases and Sciences. 49 (2): 202–9. doi:10.1023/b:ddas.0000017439.60943.5c. PMID 15104358. S2CID 31756608.
- Zhang S, Qing Q, Bai Y, Mao H, Zhu W, Chen Q, Zhang Y, Chen Y (July 2013). "Rebamipide helps defend against nonsteroidal anti-inflammatory drugs induced gastroenteropathy: a systematic review and meta-analysis". Digestive Diseases and Sciences. 58 (7): 1991–2000. doi:10.1007/s10620-013-2606-0. PMID 23456504. S2CID 4887031.
- Gagliano-Jucá T, Moreno RA, Zaminelli T, Napolitano M, Magalhães AF, Carvalhaes A, Trevisan MS, Wallace JL, De Nucci G (June 2016). "Rebamipide does not protect against naproxen-induced gastric damage: a randomized double-blind controlled trial". BMC Gastroenterology. 16 (1): 58. doi:10.1186/s12876-016-0472-x. PMC 4893238. PMID 27259970.
- "Rebamipide". Drugs.com.
- "Registration Sertificate: Rebagit (rebamipide) Film-Coated Tablets" (in Russian). Russian State Register of Medicines. Retrieved 10 June 2017.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.