Propylamine
Propylamine, also known as n-propylamine, is an amine with the chemical formula CH3(CH2)2NH2.[1] It is a colorless volatile liquid.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Propan-1-amine | |
| Other names
Propylamine | |
| Identifiers | |
3D model (JSmol) |
|
| 1098243 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.149 |
| EC Number |
|
| 1529 | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1277 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H9N | |
| Molar mass | 59.112 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | fishy, ammoniacal |
| Density | 0.719 g mL−1 |
| Melting point | −83.00 °C; −117.40 °F; 190.15 K |
| Boiling point | 47 to 51 °C; 116 to 124 °F; 320 to 324 K |
| Miscible | |
| log P | 0.547 |
| Vapor pressure | 33.01 kPa (at 20 °C) |
Henry's law constant (kH) |
660 μmol Pa−1 kg−1 |
| Acidity (pKa) | 10.71 |
Refractive index (nD) |
1.388 |
| Thermochemistry | |
Heat capacity (C) |
162.51 J K−1 mol−1 |
Std molar entropy (S⦵298) |
227.44 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−101.9–−101.1 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−2.368–−2.362 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
 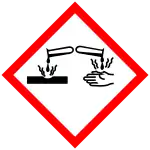  | |
| Danger | |
| H225, H302, H311, H314, H331 | |
| P210, P261, P280, P305+P351+P338, P310 | |
| Flash point | −30 °C (−22 °F; 243 K) |
| Explosive limits | 2–10.4% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Related compounds | |
Related alkanamines |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Propylamine is a weak base. Its Kb (base dissociation constant) is 4.7 × 10−4.
Preparation
Propyl amine hydrochloride can be prepared by reacting 1-propanol with ammonium chloride at high temperature and pressure using a Lewis acid catalyst such as ferric chloride.
References
- "Propylamine".
- Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a02_001
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.