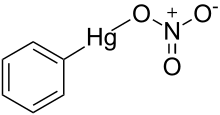
Phenylmercuric nitrate
Phenylmercuric nitrate is an organomercury compound with powerful antiseptic and antifungal effects.[1] It was once commonly used as a topical solution for disinfecting wounds, but as with all organomercury compounds it is highly toxic, especially to the kidneys, and is no longer used in this application. However it is still used in low concentrations as a preservative in eye drops for ophthalmic use, making it one of the few organomercury derivatives remaining in current medical use.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
nitrooxy(phenyl)mercury | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.221 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1895 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5HgNO3 | |
| Molar mass | 339.702 g/mol |
| Melting point | 176–186 °C (349–367 °F; 449–459 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Toxic |
| GHS labelling: | |
    | |
| Danger | |
| H301, H314, H372, H410 | |
| P260, P264, P270, P273, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P314, P321, P330, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Xu, Y.; He, Y.; Li, X.; Gao, C.; Zhou, L.; Sun, S.; Pang, G. (2013). "Antifungal effect of ophthalmic preservatives phenylmercuric nitrate and benzalkonium chloride on ocular pathogenic filamentous fungi". Diagnostic Microbiology and Infectious Disease. 75 (1): 64–7. doi:10.1016/j.diagmicrobio.2012.09.008. PMID 23102555.
- Kaur, I. P.; Lal, S.; Rana, C.; Kakkar, S.; Singh, H. (2009). "Ocular preservatives: Associated risks and newer options". Cutaneous and Ocular Toxicology. 28 (3): 93–103. doi:10.1080/15569520902995834. PMID 19505226. S2CID 9806996.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.