PRDX4
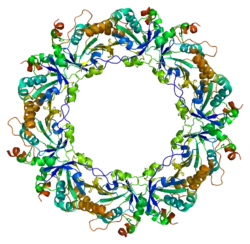
Peroxiredoxin-4 is a protein that in humans is encoded by the PRDX4 gene.[5][6] It is a member of the peroxiredoxin family of antioxidant enzymes.
Function
The protein encoded by this gene is an antioxidant enzyme of the peroxiredoxin family. The protein is localized to the cytoplasm. Peroxidases of the peroxiredoxin family reduce hydrogen peroxide and alkyl hydroperoxides to water and alcohol with the use of reducing equivalents derived from thiol-containing donor molecules. This protein has been found to play a regulatory role in the activation of the transcription factor NF-kappaB.[6]
Interactions
PRDX4 has been shown to interact with Peroxiredoxin 1.[5]
References
- GRCh38: Ensembl release 89: ENSG00000123131 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000025289 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Jin DY, Chae HZ, Rhee SG, Jeang KT (Jan 1998). "Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation". J. Biol. Chem. 272 (49): 30952–61. doi:10.1074/jbc.272.49.30952. PMID 9388242.
- "Entrez Gene: PRDX4 peroxiredoxin 4".
Further reading
- Sasagawa I, Matsuki S, Suzuki Y, Iuchi Y, Tohya K, Kimura M, Nakada T, Fujii J (2001). "Possible involvement of the membrane-bound form of peroxiredoxin 4 in acrosome formation during spermiogenesis of rats". Eur. J. Biochem. 268 (10): 3053–61. doi:10.1046/j.1432-1327.2001.02200.x. PMID 11358524.
- Wagner E, Luche S, Penna L, Chevallet M, Van Dorsselaer A, Leize-Wagner E, Rabilloud T (2002). "A method for detection of overoxidation of cysteines: peroxiredoxins are oxidized in vivo at the active-site cysteine during oxidative stress". Biochem. J. 366 (Pt 3): 777–85. doi:10.1042/BJ20020525. PMC 1222825. PMID 12059788.
- Shen C, Nathan C (2002). "Nonredundant antioxidant defense by multiple two-cysteine peroxiredoxins in human prostate cancer cells". Mol. Med. 8 (2): 95–102. doi:10.1007/BF03402079. PMC 2039972. PMID 12080185.
- Leonard D, Ajuh P, Lamond AI, Legerski RJ (2003). "hLodestar/HuF2 interacts with CDC5L and is involved in pre-mRNA splicing". Biochem. Biophys. Res. Commun. 308 (4): 793–801. CiteSeerX 10.1.1.539.8359. doi:10.1016/S0006-291X(03)01486-4. PMID 12927788.
- Ahmed M, Forsberg J, Bergsten P (2005). "Protein profiling of human pancreatic islets by two-dimensional gel electrophoresis and mass spectrometry". J. Proteome Res. 4 (3): 931–40. doi:10.1021/pr050024a. PMID 15952740.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Guo D, Han J, Adam BL, Colburn NH, Wang MH, Dong Z, Eizirik DL, She JX, Wang CY (2005). "Proteomic analysis of SUMO4 substrates in HEK293 cells under serum starvation-induced stress". Biochem. Biophys. Res. Commun. 337 (4): 1308–18. doi:10.1016/j.bbrc.2005.09.191. PMID 16236267.
- Giguère P, Turcotte ME, Hamelin E, Parent A, Brisson J, Laroche G, Labrecque P, Dupuis G, Parent JL (2007). "Peroxiredoxin-4 interacts with and regulates the thromboxane A(2) receptor". FEBS Lett. 581 (20): 3863–8. doi:10.1016/j.febslet.2007.07.011. PMID 17644091.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.