Genlisea
Genlisea (/ˌdʒɛnlɪˈsiːə/ JEN-liss-EE-ə) is a genus of carnivorous plants also known as corkscrew plants. The 30 or so species grow in wet terrestrial to semi-aquatic environments distributed throughout Africa and Central and South America. The plants use highly modified underground leaves to attract, trap and digest minute microfauna, particularly protozoans. Although suggested a century earlier by Charles Darwin, carnivory in the genus was not proven until 1998.[1]
| Genlisea | |
|---|---|
 | |
| Genlisea violacea leaves: green above-ground leaves and colorless underground trap leaves | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Lamiales |
| Family: | Lentibulariaceae |
| Genus: | Genlisea A.St.-Hil. (1833) |
| Subgenera and sections | |
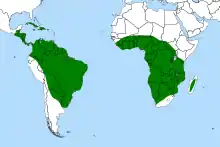 | |
| Global distribution of Genlisea | |
The generic name Genlisea honors the French writer and educator Stéphanie Félicité Ducrest de St-Albin, comtesse de Genlis.[2]
Several species in the genus, including G. margaretae, G. aurea, and G. tuberosa, possess the smallest known genomes of all flowering plants.[3][4][5]
As stated, Genlisea has a wide range of genetic diversity which can be shown in various phenotypic traits. For example, G. tuberosa develops tubers, one to three occurring per plant. This allows for carbohydrate and water storage as it is found in areas prone to fire. Other species present with a thickened stolon.[6]
Description

Genlisea are small herbs, growing from a slender rhizome and bearing two morphologically distinct leaf types - photosynthetic foliage leaves aboveground and highly modified subterranean leaves used to trap prey. The plants lack roots, although the subterranean traps perform many of the functions normally performed by roots, such as anchorage and absorption of water and nutrients.
Several to many flowers are held by a slender, erect, and often tall inflorescence. As in other members of the bladderwort family, the corolla is fused into a bilobed tube tapering to a spur, with the lower lip of the corolla having three lobes.[2] The calyx is five-lobed, in contrast to Utricularia's three-lobed calyx.[7] Corolla colors are generally yellow or violet to mauve, although a few species are white or cream.[7] The lower lip forms a palate that functions as the guide to the spur that contains the nectar by providing olfactory and mechanical stimuli for nearby pollinators like bees and flies. At a microscopic level, the palate has glandular trichomes,[8] which are small hairs that store and secrete secondary metabolites in order to provide protection from herbivory.[9] The glandular trichomes contain no nectar secretion, suggesting that they are scent glands.[8] These above ground structures are not shown to be directly participating in carnivorous activities.
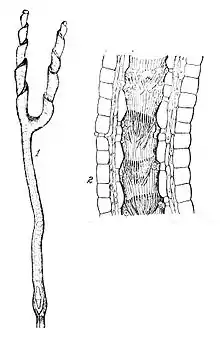
Depending on species, the photosynthetic leaves are linear to spatulate in shape and 0.5–5 cm (1⁄4–2 in) in length.[2]
The subterranean traps are white, lacking chlorophyll or any other pigmentation. They consist of a cylindrical stalk, widening at some distance below the surface into a hollow bulb-like utricle, and continuing as a hollow cylinder some further distance. At this point the stalk bifurcates into two furrowed spirals, between which the cylinder opening acts as the trap entrance. The furrows of the spiraled trap arms are lined with hairs pointing inward and toward the bifurcation. The hollow cylinder section leading from the bifurcation to the utricle is likewise lined with upward-pointing curved hairs. Some species produce two trap forms, one shorter and one longer, which probably target different prey groups. These corkscrew-like structures passively capture protozoa and other metazoan. Since these traps occur in soil, they are continuously stimulated due to the vast microfauna found in the soil. Due to continuous stimulation, the plant actively secretes digestive enzymes in order to aid with digestion to gain more nitrogen, phosphorus, and other minerals.[10] Phosphatase has been detected in all types of glands, allowing for the breakdown of prey and uptake of phosphorus in low-nutrient soils. Although not completely understood, the oxygen concentration inside Genlisea traps is negligible. For this reason, it is thought that anoxic conditions could be the mechanisms in which prey are killed. At the same time, anaerobic bacteria survive.[11]
Taxonomy
Twenty-nine species are currently recognised in the genus.[12] Two varieties are also considered valid: G. aurea var. minor and the autonymous G. aurea var. aurea.[12] Intraspecific determination depends almost wholly upon the inflorescence, particularly upon the indumentum.[7] Genetic variation is an interesting area of attention as it has an incredible high rate of nucleotide substitution rates across nucleus, chloroplast, and mitochondria when compared to other angiosperms. Due to a mutation leading to changes in phenotypic traits found in the mitochondria, reactive oxygen species are produced which ultimately lead to DNA damage and further mutations.[13]
| Species | Authority | Year | Image | Distribution | Subgenus | Section | Genome size (Mbp)[5] |
|---|---|---|---|---|---|---|---|
| Genlisea africana | Oliv. | 1865 | Africa | Genlisea | Africanae | 740 | |
| Genlisea angolensis | R.D.Good | 1924 | Africa | Genlisea | Africanae | - | |
| Genlisea aurea | A.St.-Hil. | 1833 |  | South America | Genlisea | Genlisea | 64 & 117 - 131 |
| Genlisea barthlottii | S.Porembski, Eb.Fisch. & Gemmel | 1996 | Africa | Genlisea | Africanae | - | |
| Genlisea exhibitionista[14] | Rivadavia & A.Fleischm. | 2011 | South America | Tayloria | - | ||
| Genlisea filiformis | A.St.-Hil. | 1833 |  | South America, Central America, Cuba | Genlisea | Genlisea | - |
| Genlisea flexuosa[14] | Rivadavia, A.Fleischm. & Gonella | 2011 | South America | Tayloria | - | - | |
| Genlisea glabra | P.Taylor | 1967 | South America | Genlisea | Genlisea | - | |
| Genlisea glandulosissima | R.E.Fr. | 1916 | Africa | Genlisea | Recurvatae | 154-189 | |
| Genlisea guianensis | N.E.Br. | 1900 | South America | Genlisea | Genlisea | 289 | |
| Genlisea hispidula | Stapf | 1904 |  | Africa | Genlisea | Africanae | 1510 - 1550 |
| Genlisea lobata | Fromm | 1989 |  | South America | Tayloria | - | 1200 - 1722 |
| Genlisea margaretae | Hutch. | 1946 | Africa, Madagascar | Genlisea | Recurvatae | 113 - 195 | |
| Genlisea metallica[14] | Rivadavia & A.Fleischm. | 2011 | South America | Tayloria | - | 1057 | |
| Genlisea nebulicola[14] | Rivadavia, Gonella & A.Fleischm. | 2011 | South America | Tayloria | - | - | |
| Genlisea nigrocaulis | Steyerm. | 1948 | South America | Genlisea | Genlisea | 73 - 86 | |
| Genlisea oligophylla[14] | Rivadavia & A.Fleischm. | 2011 | South America | Tayloria | - | - | |
| Genlisea oxycentron | P.Taylor | 1954 | South America, Trinidad | Genlisea | Genlisea | 75 | |
| Genlisea pallida | Fromm & P.Taylor | 1985 | Africa | Genlisea | Recurvatae | - | |
| Genlisea pulchella | Tutin | 1934 | South America | Genlisea | Genlisea | - | |
| Genlisea pygmaea | A.St.-Hil. | 1833 |  | South America | Genlisea | Genlisea | 161 - 179 |
| Genlisea repens | Benj. | 1847 |  | South America | Genlisea | Genlisea | 77 - 86 & 142 - 150 |
| Genlisea roraimensis | N.E.Br. | 1901 | South America | Genlisea | Genlisea | - | |
| Genlisea sanariapoana | Steyerm. | 1953 | South America | Genlisea | Genlisea | - | |
| Genlisea stapfii | A.Chev. | 1912 | Africa | Genlisea | Africanae | - | |
| Genlisea subglabra | Stapf | 1906 |  | Africa | Genlisea | Africanae | 1471 - 1622 |
| Genlisea tuberosa[15] | Rivadavia, Gonella & A.Fleischm. | 2013 | South America | Genlisea | Genlisea | 61[4] | |
| Genlisea uncinata | P.Taylor & Fromm | 1983 | South America | Tayloria | - | 995 - 1062 | |
| Genlisea violacea | A.St.-Hil. | 1833 |  | South America | Tayloria | - | 1005 - 1609 |
Botanical history
The genus was discovered by Augustin François César Prouvençal de Saint-Hilaire,[2] who in 1833 described four species: G. aurea, G. filiformis, G. pygmaea, and G. violacea.
Genome size range
The genus has a 25-fold range in genome size among its species and notably includes some of the smallest known plant genomes.[5] For example, the genome of G. nigrocaulis is 86 Mbp (1C; 2n = 40) while that of its close relative G. hispidula (1C; 2n = 40) is 1550 Mbp, 18-fold larger. More than one genome size has been measured in G. aurea and G. repens, suggesting that di- and tetraploid individuals exist.[5]
Host–microbiome interactions
Genlisea traps host a microbe community of bacteria (dominant species consisting of anaerobic Clostridium sp. and pectolytic Dickeya sp.[16]), green algae, microbial fungi, protists of SAR group, and minute metazoans. Through extensive research, the trap's bacterial community has been discovered as serving the ecological importance of being prey due to Genlisea plants relying on the digestive enzymatic systems from microbes in order to aid their own carnivorous digestion.[17]
References
- Barthlott W, Porembski S, Fischer E, Gemmel B (1998). "First protozoa-trapping plant found". Nature. 392 (6675): 447. doi:10.1038/33037. PMID 9548248.
- Claudi-Magnussen G (1982). "An introduction to Genlisea". Carnivorous Plant Newsletter. 11 (1): 13–15.
- Greilhuber J, Borsch T, Müller K, Worberg A, Porembski S, Barthlott W (2006). "Smallest angiosperm genomes found in Lentibulariaceae, with chromosomes of bacterial size". Plant Biology. 8: 770–777. doi:10.1055/s-2006-924101. PMID 17203433.
- Fleischmann A, Michael TP, Rivadavia F, Sousa A, Wang W, Temsch EM, Greilhuber J, Müller KF, Heubl G (2014). "Evolution of genome size and chromosome number in the carnivorous plant genus Genlisea (Lentibulariaceae), with a new estimate of the minimum genome size in angiosperms". Annals of Botany. 114 (8): 1651–1663. doi:10.1093/aob/mcu189. PMC 4649684. PMID 25274549.
- Vu, Giang; Schmutzer, Thomas; Bull, Fabian; Cao, Hieu; Fuchs, Jörg; Schubert, Ingo; others, and (2015). "Comparative Genome Analysis Reveals Divergent Genome Size Evolution in a Carnivorous Plant Genus". The Plant Genome. 8 (3): 1–14. doi:10.3835/plantgenome2015.04.0021. Retrieved 30 November 2018.
- Matos, Ramon Guedes; Silva, Saura Rodrigues; Płachno, Bartosz J.; Adamec, Lubomír; Michael, Todd P.; Varani, Alessandro Mello; Miranda, Vitor F.O. (May 2022). "The complete mitochondrial genome of carnivorous Genlisea tuberosa (Lentibulariaceae): Structure and evolutionary aspects". Gene. 824: 146391. doi:10.1016/j.gene.2022.146391. ISSN 0378-1119.
- Taylor P (1991). "The genus Genlisea". Carnivorous Plant Newsletter. 20 (1–2): 20–26.
- Płachno, Bartosz J.; Silva, Saura R.; Świątek, Piotr; Dixon, Kingsley W.; Lustofin, Krzystof; Seber, Guilherme C.; Miranda, Vitor F. O. (2020-07-21). "Structural Features of Carnivorous Plant (Genlisea, Utricularia) Tubers as Abiotic Stress Resistance Organs". International Journal of Molecular Sciences. 21 (14): 5143. doi:10.3390/ijms21145143. ISSN 1422-0067.
- Płachno, Bartosz J.; Świątek, Piotr; Stpiczyńska, Małgorzata; Miranda, Vitor Fernandes Oliveira (2018-02-14). "Flower palate ultrastructure of the carnivorous plant Genlisea hispidula Stapf with remarks on the structure and function of the palate in the subgenus Genlisea (Lentibulariaceae)". Protoplasma. 255 (4): 1139–1146. doi:10.1007/s00709-018-1220-6. ISSN 0033-183X.
- Cao, Hieu X.; Schmutzer, Thomas; Scholz, Uwe; Pecinka, Ales; Schubert, Ingo; Vu, Giang T. H. (2015-07-14). "Metatranscriptome analysis reveals host-microbiome interactions in traps of carnivorous Genlisea species". Frontiers in Microbiology. 6. doi:10.3389/fmicb.2015.00526. ISSN 1664-302X.
- Adamec, Lubomír (2007-08-23). "Oxygen Concentrations Inside the Traps of the Carnivorous Plants Utricularia and Genlisea (Lentibulariaceae)". Annals of Botany. 100 (4): 849–856. doi:10.1093/aob/mcm182. ISSN 1095-8290.
- Fleischmann, A. (2012). Monograph of the Genus Genlisea. Redfern Natural History Productions, Poole. ISBN 978-190-878-700-2.
- Carmesin, Cora F.; Fleischmann, Andreas S.; Klepsch, Matthias M.; Westermeier, Anna S.; Speck, Thomas; Jansen, Steven; Poppinga, Simon (December 2021). "Structural gradients and anisotropic hydraulic conductivity in the enigmatic eel traps of carnivorous corkscrew plants (Genlisea spp.)". American Journal of Botany. 108 (12): 2356–2370. doi:10.1002/ajb2.1779. ISSN 0002-9122.
- Fleischmann, A., F. Rivadavia, P.M. Gonella & G. Heubl (2011). A revision of Genlisea subgenus Tayloria (Lentibulariaceae). Phytotaxa 33: 1–40. first page
- Rivadavia F, Gonella PM, Fleischmann A (2013). "A new and tuberous species of Genlisea (Lentibulariaceae) from the campos rupestres of Brazil". Systematic Botany. 38 (2): 464–470. doi:10.1600/036364413X666679.
- Caravieri, Fernanda A.; Ferreira, Almir J.; Ferreira, Anderson; Clivati, Débora; de Miranda, Vitor Fernandes O.; Araújo, Welington L. (May 2014). "Bacterial community associated with traps of the carnivorous plants Utricularia hydrocarpa and Genlisea filiformis". Aquatic Botany. 116: 8–12. doi:10.1016/j.aquabot.2013.12.008. ISSN 0304-3770.
- Cao, Hieu X.; Schmutzer, Thomas; Scholz, Uwe; Pecinka, Ales; Schubert, Ingo; Vu, Giang T. H. (2015-07-14). "Metatranscriptome analysis reveals host-microbiome interactions in traps of carnivorous Genlisea species". Frontiers in Microbiology. 6. doi:10.3389/fmicb.2015.00526. ISSN 1664-302X.
- Płachno BJ, Kozieradzka-Kiszkurno M, Świątek P (2007). "Functional ultrastructure of Genlisea (Lentibulariaceae) digestive hairs". Annals of Botany. 100 (2): 195–203. doi:10.1093/aob/mcm109. PMC 2735322. PMID 17550910.
External links
- The Carnivorous Plant Society Full Carnivorous plant list.