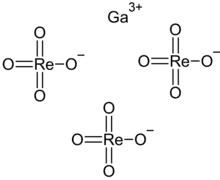
Gallium perrhenate
Gallium perrhenate is an inorganic compound with the chemical formula of Ga(ReO4)3. It exists in the anhydrous and hydrate forms.
 | |
| Names | |
|---|---|
| IUPAC name
Gallium rhenate(VII) | |
| Other names
Gallium metaperrhenate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| |
| |
| Properties | |
| Ga(ReO4)3 | |
| Molar mass | 820.344 |
| Appearance | White deliquescent crystals (anhydrous)[1] |
| Soluble | |
| Related compounds | |
Other anions |
Gallium nitrate Gallium perchlorate |
Other cations |
Potassium perrhenate |
Related compounds |
Rhenium(VII) oxide Perrhenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Gallium can be anodically dissolved in perrhenic acid at 50-55 °C, and dried with sulfuric acid to obtain the hydrate crystal [Ga(H2O)6(ReO4)3]·2H2O.[2] The anhydrous form can be obtained by reacting gallium(III) oxide and rhenium(VII) oxide at 450 °C.[1]
Chemical properties
Gallium perrhenate decomposes above 300 °C to form rhenium(VII) oxide and gallium(III) oxide:[3]
- 2 Ga(ReO4)3 → 3 Re2O7 + Ga2O3
References
- Baud, Gilbert; Capestan, Michel (1968). "Gallium perrhenate and its hydrates". Comptes Rendus de l'Académie des Sciences, Série C (in French). 266 (6): 382–384. ISSN 0567-6541.
- Vol'fkovich, A. Yu.; Khrustalev, V. N.; Shamrai, N. B.; Varfolomeev, M. B. (1997). "Electrochemical synthesis of aluminum, gallium, and indium perrhenates". Zhurnal Neorganicheskoi Khimii (in Russian). 42 (12): 1960–1962. ISSN 0044-457X.
- 谢高阳 等主编 (ed.). "3.13.3 含氧酸及其盐类". 第九卷 锰分族 铁系 铂系 [Volume IX Manganese Group Iron Series Platinum Series]. 无机化学丛书. 科学出版社. p. 116.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.