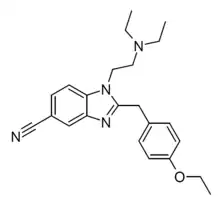
Etonitazene 5-cyano analogue
Etonitazene 5-cyano analogue (Etocyanazene, 5-cyanodesnitroetonitazene) is a benzimidazole derivative with opioid effects, first developed in the 1950s as part of the research that led to better-known compounds such as etonitazene. It is an analogue of etonitazene where the 5-nitro (NO2) group has been replaced by a nitrile (C≡N) group.[1] It is described as having "reduced but still significant" potency compared to etonitazene itself.[2] It was made illegal in Germany in July 2021.[3]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C23H28N4O |
| Molar mass | 376.504 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Lecolier S, Trouiller G (1967). "Nouveaux benzimidazoles doués d'activité morphinique" [New benzimidazoles with opioid activity.]. Chim. Ther. (in French). 2: 16–24.
- "A review of the evidence on the use and harms of 2-benzyl benzimidazole ('nitazene') and piperidine benzimidazolone ('brorphine-like') opioids" (PDF). UK: Advisory Council on the Misuse of Drugs. July 2022.
- "Anhang - Zweite Verordnung zur Änderung der Anlage des Neue-psychoaktive-Stoffe-Gesetzes" [Appendix - Second regulation amending the appendix to the New Psychoactive Substances Act]. Gesetze und Verordnungen des deutschen Bundesrechts im Internet [Laws and regulations of German federal law on the Internet] (in German).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.