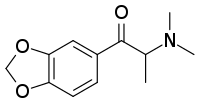
Dimethylone
Dimethylone (βk-MDDMA) is a substituted cathinone derivative with stimulant and empathogenic effects. Unlike the corresponding amphetamine derivative MDDM which is thought to be practically inactive, dimethylone substitutes for methamphetamine and MDMA in animal studies and has been sold as a designer drug.[2][3][4][5]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Legality
In the United States Dimethylone is considered a schedule 1 controlled substance as a positional isomer of Butylone[6]
See also
References
- Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Zaitsu K, Katagi M, Kamata HT, Miki A, Tsuchihashi H (2008). "Discrimination and identification of regioisomeric β-keto analogues of 3,4-methylenedioxyamphetamines by gas chromatography-mass spectrometry". Forensic Toxicol. 26 (2): 45–51. doi:10.1007/s11419-008-0050-1. S2CID 2871089.
- Kikura-Hanajiri R, Uchiyama N, Goda Y (May 2011). "Survey of current trends in the abuse of psychotropic substances and plants in Japan". Legal Medicine. 13 (3): 109–15. doi:10.1016/j.legalmed.2011.02.003. PMID 21377397.
- Krotulski AJ, Mohr AL, Fogarty MF, Logan BK (October 2018). "The Detection of Novel Stimulants in Oral Fluid from Users Reporting Ecstasy, Molly and MDMA Ingestion". Journal of Analytical Toxicology. 42 (8): 544–553. doi:10.1093/jat/bky051. PMID 30371847.
- Gatch MB, Dolan SB, Forster MJ (June 2019). "Locomotor activity and discriminative stimulus effects of five novel synthetic cathinone analogs in mice and rats". Drug and Alcohol Dependence. 199: 50–58. doi:10.1016/j.drugalcdep.2019.02.016. PMC 6534427. PMID 30986635.
- https://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.