Development of COVID-19 tests
| Part of a series on the |
| COVID-19 pandemic |
|---|
 Scientifically accurate atomic model of the external structure of SARS-CoV-2. Each "ball" is an atom. |
|
|
|
Introduction
The COVID-19 transmission is rapid and occurs via inhalation of respiratory droplets or aerosol particles contaminated with SARS-CoV-2. The viral deposition occurs on mucous membranes of nose, mouth, or eyes via sprays and splashes; or direct contact with hands contaminated with SARS-CoV-2 either by respiratory fluids or by touching surfaces contaminated with the virus. Evidence from studies suggests that SARS-CoV-2 aerosols can stay viable in air for about 3 hours, have a half-life of about 1 hour and can survive for more time on steel and plastic while compared to cardboard and copper. Hence, developing a therapy against COVID-19 was progressing at a pandemic speed, yet there is no effective therapy approved by food and drug administration (FDA). Vaccines seem promising and currently include Pfizer-BioNTech vaccine, Moderna vaccine, Oxford/AstraZeneca vaccine, and the Janssen vaccine. But all of these therapeutic strategies cannot offer complete protection against COVID-19 paving way for epidemiological approaches to break the chain of disease transmission. In such an approach, diagnostics is the most crucial tool helping to identify the disease cases leading to isolation as well as contact tracing, thereby preventing other people from coming in contact with the patients or their recent contacts.[2]
The development of COVID-19 tests was a major public health priority during the early months of the COVID-19 pandemic. In January 2020, scientists from China published the first genetic sequences of SARS-CoV-2 via virological.org,[3] a "hub for prepublication data designed to assist with public health activities and research".[4] Researchers around the world used that data to build molecular tests for the virus. Antigen- and antibody-based tests were developed later.
Even once the first tests were created, the supply was limited. As a result, no country had reliable data on the prevalence of virus early in the pandemic.[5] The WHO and other experts called for ramping up testing as the best way to slow the spread of the virus.[6][7] Shortages of reagent and other testing supplies became a bottleneck for mass testing in the EU, the UK and the US.[8][9][10] Early tests also encountered problems with reliability.[11][12]
2020
January
Public Health England announced a test on the 10th,[13] using a real-time RT-PCR (RdRp gene) assay based on oral swabs.[14] The test detected the presence of any type of coronavirus, including specifically identifying SARS-CoV-2. It was rolled out to twelve laboratories across the United Kingdom on 10 February.[15]
Scientists from China first released information on the viral genome on 10 January 2020,[4][16]. That day the Malaysian Institute for Medical Research (IMR) produced "primers and probes" specific to a SARS-CoV-2 RT-PCR test.[17] The IMR's materials were used to diagnose Malaysia's first patient on 24 January.[18] BGI Group was one of the first companies to receive emergency use approval from China's National Medical Products Administration for a nucleic acid test.[19]
The German nucleic acid testing protocol was published on the 17th. Another early PCR test was developed by Charité University hospital in Berlin, working with academic collaborators in Europe and Hong Kong, and published on the 23rd. It used rtRT-PCR, and formed the basis of 250,000 kits distributed by the World Health Organization (WHO).[20]
In Russia, the first COVID‑19 test was developed by the State Research Center of Virology and Biotechnology VECTOR. Production began on 24 January.[21]
In the US, the Centers for Disease Control and Prevention (CDC) developed its SARS-CoV-2 Real Time PCR Diagnostic Panel.[22] The protocol became available on the 28th.[23] One of three tests in early kits failed due to faulty reagents.[11]
February
South Korean company Kogenebiotech's clinical grade, nucleic acid test (PowerChek Coronavirus) was approved by Korea Centers for Disease Control and Prevention (KCDC) on 4 February.[24]
In Wuhan, BGI opened a makeshift 2000-sq-meter emergency detection laboratory named "Huo-Yan" (Chinese: 火眼, "Fire Eye") on the 5th.[25][26] It processed more than 10,000 samples/day.[26][27] Construction required 5 days.[28] The Wuhan Laboratory was followed by Huo-Yan labs in Shenzhen, Tianjin, Beijing, and Shanghai, in a total of 12 cities across China.
On 11 February, the test was approved by the Federal Service for Surveillance in Healthcare in Russia.[29]
In the United States, the CDC refused to let other labs process tests that month, allowing an average of fewer than 100 samples/day to be processed. Tests using two components were not determined to be reliable until the 28th, and only then were state and local laboratories permitted to begin testing.[30] The test was approved by the FDA under an EUA.
March
Due to limited testing, no countries had reliable data on the prevalence of the virus in their population.[5] Testing variability distorts reported case fatality rates, which were probably overestimated in many countries due to sampling bias.[31][32] Shortages of reagent and other supplies became a bottleneck for mass testing in the EU and UK[8] and the US.[9][10]
By 4 March, China reached 50,000 tests per day.[33] A study examined 1070 samples from 205 Wuhan patients and reported varied sensitivity according to the methods and location of sample collection. Samples from bronchoalveolar lavage fluid specimens returned the highest sensitivity.[34] The authors argued that CT scans showed even higher sensitivity.[35]
US commercial labs began testing in early March. As of the 5th, LabCorp announced nationwide availability of COVID‑19 testing based on RT-PCR.[36] Quest Diagnostics made nationwide testing available as of 9 March.[37] US testing demand grew rapidly, causing backlogs of hundreds of thousands of tests at private US labs. Supplies of swabs and chemical reagents continued strained.[38] On 25 May, the US required each state to take responsibility for meeting its testing needs.[39] In March, the FDA issued EUAs for nucleic acid tests to Hologic (3/16),[40] Abbott Laboratories (3/18),[41] Thermo Fisher Scientific (3/19)[42] Cepheid (3/21)[43][44] and LabCorp (4/30).[41]
On 12 March, Mayo Clinic announced a nucleic acid test.[45]
On 16 March, the WHO called for ramping up testing programmes as the best way to slow the spread.[6][7] Several European countries initially conducted more tests than the US.[46][47] By 19 March, drive-in tests were offered in several large cities.[48]
As of 22 March, according to the president of the Robert Koch Institute, Germany had capacity for 160,000 tests per week.[49] As of 26 March, German Health Minister Jens Spahn estimated that Germany was conducting 200,000 tests per week.[50] Germany has a large medical diagnostics industry, with more than a hundred testing labs that provided the technology and infrastructure to enable rapid increases in testing. Costs are borne by insurance when the test is ordered by a physician.[51] As of the end of March at least 483,295 samples were tested and 33,491 (6.9%) had tested positive.[52]
On 26 March, it was reported that 80% of test kits that Czechia purchased from China gave inaccurate results.[53][54] Slovakia purchased 1.2 million antibody-based test kits from China that were found to be inaccurate.[55] China accused Czechia and Slovakia of incorrect use of those tests.[56] Ateş Kara of the Turkish Health Ministry said the test kits Turkey purchased from China had a "high error rate".[57][58]
Spain purchased test kits from Chinese firm Shenzhen Bioeasy Biotechnology Co Ltd, but found that results were unacceptable. The maker explained that the incorrect results may stem from failure to collect samples or use the kits correctly. On 27 March, the Spanish ministry switched to another vendor, Shenzhen Bioeasy.[59]
By 31 March, the United Arab Emirates was testing more of its population per head than any other country.[60] UAE implemented a combination of drive-through sample collection, and a mass-throughput laboratory from Group 42 and BGI. The lab conduced tens of thousands RT-PCR tests per day and was the first to be operational at that scale other than China.[61]
By the month's end, testing had surpassed 200k/week.[62]
April
The FDA gave an EUA for the US' first antibody test on the 2nd.[63][64]
On 5 April, the U.S. subsidiary of China's BGI Group sent a proposal to the state of California offering to build in California, at cost ($10 million), the world's largest COVID-19 testing site, in two weeks, and train Americans to operate it. California's consultants recommended against it, because of the risk of security and commercial competition.[65]
As of 7 April, the World Health Organization (WHO) had accepted two diagnostic tests for procurement under the Emergency Use Listing procedure (EUL).[66]
On 13 April, Health Canada approved a nucleic acid test from Spartan Bioscience. Institutions may "test patients" with a handheld DNA analyzer "and receive results without having to send samples away to a [central] lab".[67][68]
By the start of April, the United Kingdom was delivering around 10,000 swab tests per day.[69] The British NHS announced that it was piloting a scheme to test suspected cases at home, to remove the risk of one patient infecting others at a hospital or disinfecting an ambulance used to transport a patient.[70]
The UK purchased 3.5 million antibody test kits from China, but in early April 2020 announced these were not usable.[71][72] On 21 April 2020, the Indian Council of Medical Research (ICMR) advised Indian states to stop using test kits purchased from China after receiving complaints from one state. Rajasthan health minister Raghu Sharma on 21 April said the kits gave only 5.4 percent accurate results.[73]
Antibody survey results found from 2% to 30% positive.[74] On preliminary data, WHO concluded that 2% to 3% of the world population had developed antibodies.[75]
By month end, testing had surpassed 750k/week.[62]
May
In May antibody tests were conducted on 5,603 major league baseball employees and 0.7% tested positive, showing that they had been infected. 70% of those who tested positive had had no symptoms.[76][77][78] The US was conducting an average of 2.5 million tests per week for the week ending 17 May. This grew to 3.2 million by 14 June.[79][80]
Attempts to culture virus from upper respiratory specimens were largely unsuccessful when viral burden is low but detectable (i.e., cycle threshold values higher than 33–35).[81]
On 1 May, Quotient Limited announced the CE Mark for its MosaiQ COVID-19 antibody test,[82] designed as a serological disease screen specific to the Coronavirus.[83] The test has a 100% sensitivity and 99,8% specificity claim.[84][85]
On 3 May, Roche received an EUA for a selective ELISA serology test.[86][87]
On 8 May, the FDA granted its first EUA for antigen test: "Sofia 2 SARS Antigen FIA" by Quidel Corp.[88][89]
The FDA announced on 14 May a review of 15 adverse event reports about the Abbott ID Now device for low sensitivity.[90]
On 21 May, researchers at Ben-Gurion University in Israel reported a one-minute coronavirus test with 90% accuracy, based on the "change in the resonance in the THz spectral range" shown by the coronavirus through THz spectroscopy.[91]
Nearly two million antibody tests imported into Australia and costing $20 million were declared unusable.[92][93][94]
In early May Harvard's Global Health Institute estimated that the US needed to test more than 900k per day.[95][96] Other recommendations ranged up to 23m per day.[97][98][99][100]
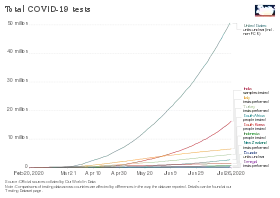
As of 24 May, countries that publicised their testing data had typically performed tests equal to 2.6 percent of their population, although no country had tested more than 17.3%.[101]
On 29 May Siemens received an EUA for its anti-spike RBD-targeting serology test that it believes detects neutralizing antibodies.[102]
By month end, testing had surpassed 1.4m/week.[62]
June
In June, researchers announced a nucleic acid diagnostic test using reverse transcription-loop-mediated isothermal amplification (RT-LAMP), an existing technology used in pathogenic microorganism identification, genetically modified ingredients, tumor detection, and embryo sex identification. The test identified virus in samples of serum, urine, saliva, oropharyngeal swabs and nasopharyngeal swabs. Once commercialized the test has the potential to provide rapid (30-45 minute) diagnosis at point of care. The test was 100% selective and highly sensitive, detecting virus at a concentration of .06 fg/ml.[103]
As of 14 June 2020, the percentage testing positive in the US as a whole had fallen below 5%.[104] As of late June, test numbers crossed 600k/day.[79]
November
On 6 November, the U.S. Food and Drug Administration (FDA) authorized the first serology test that detects neutralizing antibodies from recent or prior SARS-CoV-2 infection, which are antibodies that bind to a specific part of a pathogen and have been observed in a laboratory setting to decrease SARS-CoV-2 viral infection of cells.[105] The FDA issued an emergency use authorization (EUA) for the cPass SARS-CoV-2 Neutralization Antibody Detection Kit, which specifically detects this type of antibody.[105] The FDA granted Lucira Health emergency use authorization for the first US at-home rapid molecular diagnostic test. With a prescription from a healthcare provider, consumers can use the test kit to take a nasal swab then perform a 30-minute SARS-CoV-2 detection test at home.[106]
December
On 15 December, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the first over-the-counter (OTC) fully at-home diagnostic test for COVID-19.[107][108][109] The 'Ellume COVID-19 Home Test' is a rapid, lateral flow antigen test, a type of test that runs a liquid sample along a surface with reactive molecules.[107] The test detects fragments of proteins of the SARS-CoV-2 virus from a nasal swab sample from any individual two years of age or older.[107] The Ellume test uses a mid-turbinate nasal swab (sample is collected further back than the usual nasal swab, but not as far back as nasopharyngeal swabs, which are only appropriate for use by a trained health care provider) to detect certain proteins of the virus known as antigens.[107] The Ellume test uses an analyzer that connects via bluetooth with a software application on a smartphone to help users perform the test and interpret results.[107] Results are delivered in as little as 20 minutes to individuals via their smartphone.[107]
2021
July
On July 21, the U.S. CDC announced that, after December 31, 2021, it "will withdraw the request to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel" and that the CDC encourages "laboratories to consider adoption of a multiplexed method that can facilitate detection and differentiation of SARS-CoV-2 and influenza viruses."[110][111]
References
- Roser M, Ritchie H, Ortiz-Ospina E (4 March 2020). "Coronavirus Disease (COVID-19) – Statistics and Research". Our World in Data – via ourworldindata.org.
- Mathew, Bijo; Kumar, Rajesh; Harilal, Seetha; Al-Sehemi, Abdullah G.; Pannipara, Mehboobali; Mathew, Githa Elizabeth (2022-09-21). "Advancements in COVID- 19 Testing: An in-depth overview". Current Pharmaceutical Biotechnology. 23 (9): 1122–1148. doi:10.2174/1389201023666220921144150. ISSN 1389-2010. PMID 36154593. S2CID 252513275.
- "Novel 2019 coronavirus genome". Virological.org. 11 January 2020. Retrieved 12 April 2023.
- Schnirring, Lisa (11 January 2020). "China releases genetic data on new coronavirus, now deadly". CIDRAP. Archived from the original on 11 January 2020. Retrieved 12 January 2020.
- Ioannidis, John P.A. (17 March 2020). "A fiasco in the making? As the coronavirus pandemic takes hold, we are making decisions without reliable data". STAT. Retrieved 22 March 2020.
- "'Test, Test, Test': WHO Chief's Coronavirus Message to World". The New York Times. Reuters. 16 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- Farge E, Revill J (17 March 2020). "'Test, test, test': WHO chief's coronavirus message to world". Reuters. Retrieved 6 November 2020.
- "Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK" (PDF). European Centre for Disease Prevention and Control. 25 March 2020. pp. 15–16. Retrieved 29 March 2020.
the current shortages of laboratory consumables and reagents affect diagnostic capacity and hamper the epidemic response at the national and local levels. The laboratories have experienced delayed or missing deliveries of swabbing material, plastic consumables, RNA extraction and RT-PCR reagents, and PPE. This is affecting laboratories in all EU/EEA countries.
- Baird RP (24 March 2020). "Why Widespread Coronavirus Testing Isn't Coming Anytime Soon". The New Yorker. Archived from the original on 28 March 2020. Retrieved 29 March 2020.
South Dakota, said that her state's public-health laboratory—the only lab doing COVID-19 testing in the state—had so much trouble securing reagents that it was forced to temporarily stop testing altogether. also noted critical shortages of extraction kits, reagents, and test kits
- Ossola A (25 March 2020). "Here are the coronavirus testing materials that are in short supply in the US". Quartz. Archived from the original on 26 March 2020. Retrieved 29 March 2020.
extract the virus's genetic material—in this case, RNA—using a set of chemicals that usually come in pre-assembled kits. 'The big shortage is extraction kits' There are no easy replacements here: 'These reagents that are used in extraction are fairly complex chemicals. They have to be very pure, and they have to be in pure solution'
- Temple-Raston, Dina (2020-11-06). "CDC Report: Officials Knew Coronavirus Test Was Flawed But Released It Anyway". NPR. Archived from the original on 2020-11-06. Retrieved 2021-03-20.
- Armario, Christine (2020-10-07). "Peru bet heavily on cheap COVID tests; it didn't go well". Associated Press. Archived from the original on 2020-10-07. Retrieved 2021-03-20.
- "UK defends coronavirus response after Reuters investigation". Reuters. 9 April 2020. Retrieved 12 April 2020.
After developing a test for the new virus by Jan. 10
- "COVID-19 virus testing in NHS laboratories" (PDF). NHS England and NHS Improvement. 16 March 2020. Archived from the original (PDF) on 12 April 2020. Retrieved 25 March 2021.
- "PHE novel coronavirus diagnostic test rolled out across UK". GOV.UK. Retrieved 30 March 2020; "'Increased likelihood' of China virus reaching UK". BBC News. 23 January 2020. Retrieved 30 March 2020; "PHE tells patients with suspected coronavirus to call GP or NHS 111". The Pharmaceutical Journal. 27 January 2020. Retrieved 30 March 2020.
- Schnirring, Lisa (13 January 2020). "Thailand finds Wuhan novel coronavirus in traveler from China". CIDRAP. Archived from the original on 13 January 2020. Retrieved 14 January 2020.
- "Laboratory Readiness for Detecting the 2019 novel coronavirus (2019-nCoV) infection in Malaysia". Director-General of Health, Malaysia. 9 February 2020.
- "Malaysia must ramp up testing". The Star Malaysia. 26 March 2020.
- "BGI Sequencer, Coronavirus Molecular Assays Granted Emergency Use Approval in China". GenomeWeb. 29 January 2020. Retrieved 9 March 2020.
- Sheridan C (April 2020). "Coronavirus and the race to distribute reliable diagnostics". Nature Biotechnology. 38 (4): 382–84. doi:10.1038/d41587-020-00002-2. PMID 32265548. S2CID 214163420.
- "Совещание по вопросам развития ситуации с коронавирусной инфекцией и мерам по её профилактике" [Meeting on the development of the situation with coronavirus infection and measures for its prevention]. Президент России. 7 April 2020.
- "CDC Diagnostic Test for COVID-19". U.S. Centers for Disease Control and Prevention (CDC). Retrieved 15 June 2019.
- "Biden falsely says Trump administration rejected WHO coronavirus test kits (that were never offered)". PolitiFact.
- Jeong S (28 February 2020). "Korea approves 2 more COVID-19 detection kits for urgent use". Korea Biomedical Review. Retrieved 12 March 2020.
- "Wuhan Test Lab Opens; CDC Ships Diagnostic Kits: Virus Update". Bloomberg. 5 February 2020. Retrieved 7 February 2020.
- "China virus crisis deepens as whistleblower doctor dies". AFP.com. 27 February 2012. Retrieved 7 February 2020.
- 日检测量达万份的"火眼"实验室连夜试运行 [The "Huoyan" laboratory with a daily test volume of up to 10,000 test runs overnight].
- "BGI's Coronavirus Response? Build a Lab in Wuhan". GEN – Genetic Engineering and Biotechnology News. 12 February 2020. Retrieved 27 March 2020.
- "В России зарегистрирована отечественная тест-система для определения коронавируса" [A domestic test system for detecting coronavirus is registered in Russia]. Interfax-Russia.ru. 14 February 2020.
- CDC (28 February 2020). "Transcript for the CDC Telebriefing Update on COVID-19". U.S. Centers for Disease Control and Prevention (CDC) (Press release). Retrieved 6 November 2020.
- Ward, D. (April 2020) "Sampling Bias: Explaining Wide Variations in COVID-19 Case Fatality Rates". WardEnvironment. doi: 10.13140/RG.2.2.24953.62564/1
- Henriques, Martha. "Coronavirus: Why death and mortality rates differ". bbc.com. Retrieved 8 April 2020.
- "COVID-19 Local Laboratory Solution". BGI – Global. Retrieved 27 March 2020.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (March 2020). "Detection of SARS-CoV-2 in Different Types of Clinical Specimens". JAMA. 323 (18): 1843–44. doi:10.1001/jama.2020.3786. PMC 7066521. PMID 32159775.
- "Comparing RT-PCR and Chest CT for Diagnosing COVID-19". HCPLive. Archived from the original on 23 May 2020. Retrieved 23 May 2020.
- "LabCorp Launches Test for Coronavirus Disease 2019 (COVID-19) | Laboratory Corporation of America Holdings". ir.labcorp.com. Retrieved 16 June 2020.
- "Covid19 : COVID-19". questdiagnostics.com.
- Stein R (3 April 2020). "Coronavirus Testing Backlogs Continue As Laboratories Struggle To Keep Up With Demand". NPR.org. Retrieved 15 June 2020.
- Mandavilli A, Edmondson C (25 May 2020). "'This Is Not the Hunger Games': National Testing Strategy Draws Concerns". The New York Times.
- "Hologic's Molecular Test for the Novel Coronavirus, SARS-CoV-2, Receives FDA Emergency Use Authorization". Hologic. 16 March 2020. Retrieved 13 April 2020.
- "Emergency Use Authorization". FDA. 30 April 2020. Archived from the original on 29 April 2020.
- "FDA Approves Abbott Laboratories Coronavirus Test, Company To Ship 150,000 Kits". IBTimes.com. 19 March 2020. Archived from the original on 20 March 2020.
- "Sunnyvale company wins FDA approval for first rapid coronavirus test with 45-minute detection time". EastBayTimes.com. 21 March 2020. Archived from the original on 22 March 2020.
- "Xpert Xpress SARS-CoV-2 has received FDA Emergency Use Authorization". cepheid.com. Retrieved 13 April 2020.
- Plumbo G (12 March 2020). "Mayo Clinic develops test to detect COVID-19 infection". Mayo Clinic. Retrieved 13 March 2020.
- "Daily COVID-19 tests per thousand people". Our World in Data. Retrieved 15 April 2020.
- "Total tests for COVID-19 per 1,000 people". Our World in Data. Retrieved 15 April 2020.
- "Covid-19 – Tests auf das Coronavirus: Wann, wo und wie?" [Covid-19 - tests for the coronavirus: when, where and how?]. Deutschlandfunk (in German). 19 March 2020. Archived from the original on 18 April 2020. Retrieved 24 March 2020.
- Oltermann P (22 March 2020). "Germany's low coronavirus mortality rate intrigues experts". The Guardian. ISSN 0261-3077. Retrieved 24 March 2020.
- Charisius H (26 March 2020). "Covid-19: Wie gut testet Deutschland?" [Covid-19: How well is Germany testing?] (in German). Retrieved 26 March 2020.
- Weber N, Rydlink K, Irene Berres (5 March 2020). "Coronavirus und Covid-19: So testet Deutschland" [Coronavirus and Covid-19: This is how Germany tests]. Der Spiegel (in German). Retrieved 23 March 2020.
- "Coronavirus Disease 2019 Daily Situation Report of the Robert Koch Institute" (PDF). Robert Koch Institute. 26 March 2020. Retrieved 28 April 2020.
- "80% of Rapid COVID-19 Tests the Czech Republic Bought From China are Wrong". Prague Morning. 26 March 2020.
- Blažek V (23 March 2020). "Úřad dopředu psal, kdy mohou rychlotesty selhat. I tak je stát nasadil" [The office wrote in advance when rapid tests could fail. Even so, the state deployed them]. Zeznam Zprávy (in Czech). Retrieved 7 April 2020.
Indeed, the rapid tests that arrived from China a few days ago do not really reliably detect the infection at an early stage.
- DUDIK, ANDREA; TOMEK, RADOSLAV (1 April 2020). "Europe turned to China for coronavirus testing help. Why some are now regretting it". Fortune. Retrieved 28 June 2020.
- "Faulty virus tests cloud China's European outreach over COVID-19". The Jakarta Post. Retrieved 23 May 2020.
- "Coronavirus test kits purchased from China are unreliable, says Science Committee member". www.duvarenglish.com. 27 March 2020. Retrieved 28 June 2020.
- Soylu, Ragip (27 March 2020). "Coronavirus: Turkey rejects Chinese testing kits over inaccurate results". Middle East Eye. Retrieved 28 June 2020.
- "Chinese firm to replace exported coronavirus test kits deemed defective by Spain". Reuters. 27 March 2020 – via www.reuters.com.
- Sullivan H, Rawlinson K, Gayle D, Topping A, Mohdin A, Willsher K, Wintour P, Wearden G, Greenfield P (31 March 2020). "Global confirmed virus death toll passes 40,000 – as it happened". The Guardian. ISSN 0261-3077. Retrieved 1 April 2020.
- "VIDEO: UAE sets up COVID-19 detection lab in just 14 days". Gulf Today. 31 March 2020.
- "Coronavirus Pandemic Data Explorer". Our World in Data. Retrieved 28 June 2020.
- "What Immunity to COVID-19 Really Means". Scientific American. 10 April 2020. Archived from the original on 28 April 2020.
- Romano, Andrew. (7 April 2020). "Fauci once dismissed concerns about 'silent carriers' of coronavirus. Not anymore." Yahoo News Retrieved 17 April 2020.
- Jeanne Whalen and Elizabeth Dwoskin (2 July 2020). "California rejected Chinese company's push to help with coronavirus testing. Was that the right move? Advisers told the state to steer clear of BGI, underscoring U.S.-China tech tension". The Washington Post.
- "WHO lists two COVID-19 tests for emergency use". World Health Organization (WHO). Retrieved 10 April 2020.
- "Health Canada approves new rapid COVID-testing kits". The Globe and Mail Inc. 13 April 2020.
- "The power of DNA testing for everyone". Spartan Bioscience. Archived from the original on 22 April 2020. Retrieved 14 April 2020.
- "Coronavirus (COVID-19): Scaling up our testing programmes" (PDF). Department of Health and Social Care. 4 April 2020.
- "NHS pilots home testing for coronavirus". MobiHealthNews. 24 February 2020. Archived from the original on 25 February 2020.
- Kenber B (6 April 2020). Smyth C, Kennedy D (eds.). "Britain has millions of coronavirus antibody tests, but they don't work" – via www.thetimes.co.uk.
None of the antibody tests ordered by the government is good enough to use, the new testing chief has admitted. Professor John Newton said that tests ordered from China.
- "Government's testing chief admits none of 3.5m coronavirus antibody kits work sufficiently". The Independent. 6 April 2020.
- "ICMR asks states not to use rapid test kits for two days". The Telegraph. 21 April 2020.
- Vogel G (21 April 2020). "Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable". Science | AAAS. Retrieved 31 May 2020.
- "WHO Director-General's opening remarks at the media briefing on COVID-19". World Health Organization (WHO). 20 April 2020. Retrieved 31 May 2020.
- "MLB antibody study: 0.7% of those tested had been exposed to coronavirus". SFChronicle.com. 11 May 2020. Archived from the original on 19 May 2020. Retrieved 13 May 2020.
- Wagner J (15 April 2020). "M.L.B. Employees Become the Subjects of a Huge Coronavirus Study". The New York Times. Retrieved 20 May 2020.
- "Fewer than 1% of MLB employees test positive for COVID-19 antibodies". Los Angeles Times. 10 May 2020. Retrieved 20 May 2020.
- "US Historical Data". The COVID Tracking Project.
- "COVID-19: Tests per day". Our World in Data. Retrieved 15 April 2020.
- "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19". U.S. Centers for Disease Control and Prevention (CDC). 3 May 2020. Archived from the original on 4 May 2020.
- "The MosaiQ COVID-19 Antibody Microarray". Quotient. Retrieved 1 October 2020.
- "Quotient Limited Announces CE Mark For Its Sars-Cov-2 (Covid-19) Antibody Microarray". Reuters. May 2020. Retrieved 1 October 2020.
- "Coronavirus: New 'fast and accurate' antibody test developed". BBC News. 4 May 2020. Retrieved 1 October 2020.
- "Quotient Limited Gets CE Mark for Coronavirus Antibody Microarray". 360Dx. May 2020. Retrieved 1 October 2020.
- "Roche's COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark". Roche (Press release). 3 May 2020. Retrieved 8 May 2020.
- "Roche Diagnostics GmbH Elecsys Anti-SARS-CoV-2" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 8 May 2020.
- "FDA issues emergency approval of new antigen test that is cheaper, faster and simpler". The Washington Post. 9 May 2020.
- Office of the Commissioner (9 May 2020). "Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients". FDA.
- Commissioner, Office of the (14 May 2020). "Coronavirus (COVID-19) Update: FDA Informs Public About Possible Accuracy Concerns with Abbott ID NOW Point-of-Care Test". FDA. Retrieved 15 May 2020.
- Ho D (21 May 2020). "Israel's Ben-Gurion University develops one-minute coronavirus test". BioWorld.com. Retrieved 7 June 2020.
- Mannix L (13 May 2020). "'Flipping a coin': COVID-19 antibody tests 'should not be used'". The Sydney Morning Herald. Retrieved 23 May 2020.
- Mannix L (12 May 2020). "The government bought 1.5 million antibody tests. They can't be used". The Sydney Morning Herald. Retrieved 29 May 2020.
- Australian Government Department of Health Therapeutic Goods Administration (22 May 2020). "Post-market evaluation of serology-based point of care tests". Therapeutic Goods Administration (TGA). Retrieved 23 May 2020.
- "HGHI and NPR publish new state testing targets – Pandemics Explained". globalepidemics.org. 7 May 2020. Retrieved 11 May 2020.
- "U.S. Coronavirus Testing Still Falls Short. How's Your State Doing?". NPR.org. 7 May 2020. Retrieved 11 May 2020.
- "What Testing Capacity Do We Need?". The Henry J. Kaiser Family Foundation. 17 April 2020. Retrieved 11 May 2020.
- Romer P. "Roadmap to responsibly reopen America" (PDF). Retrieved 11 May 2020.
- "Roadmap to pandemic resilience" (PDF). Edmond J. Safra for Ethics at Harvard University. 20 April 2020. Retrieved 11 May 2020.
- "National Covid-19 Testing Action Plan". The Rockefeller Foundation. Retrieved 11 May 2020.
- "Total tests for COVID-19 per 1,000 people". Our World in Data. Archived from the original on 18 May 2020. Retrieved 16 April 2020.
- Tuzman, Karen Tkach (11 June 2020). "Emerging COVID-19 serology tests aim for neutralizing antibodies". BioCentury. Retrieved 26 June 2020.
- Lamb LE, Bartolone SN, Ward E, Chancellor MB (12 June 2020). "Rapid detection of novel coronavirus/Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by reverse transcription-loop-mediated isothermal amplification". PLOS ONE. 15 (6): e0234682. Bibcode:2020PLoSO..1534682L. doi:10.1371/journal.pone.0234682. PMC 7292379. PMID 32530929.
- "All State View of Week to Week Change in Percentage of Positive Tests". Johns Hopkins Coronavirus Resource Center. 15 June 2020. Retrieved 15 June 2020.
- "Coronavirus (COVID-19) Update: FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection". U.S. Food and Drug Administration (FDA) (Press release). 6 November 2020. Retrieved 6 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Erdman, Shelby. "FDA authorizes first rapid Covid-19 self-testing kit for at-home diagnosis". [CNN]. Retrieved 18 Nov 2020.
- "Coronavirus (COVID-19) Update: FDA Authorizes Antigen Test as First Over-the-Counter Fully At-Home Diagnostic Test for COVID-19". U.S. Food and Drug Administration (FDA) (Press release). 15 December 2020. Retrieved 15 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "NIH-funded COVID-19 home test is first to receive over-the-counter authorization from FDA". National Institutes of Health (NIH) (Press release). 15 December 2020. Retrieved 15 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA Authorizes Ellume COVID-19 Home Test as First Over-the-Counter Fully At-Home Diagnostic Test". Ellume Health (Press release). 15 December 2020. Retrieved 15 December 2020 – via GlobeNewswire.
- CDC (2021-07-21). "Lab Alert: Changes to CDC RT-PCR for SARS-CoV-2 Testing". www.cdc.gov. Archived from the original on 2021-07-22. Retrieved 2021-07-26.
- McFall, Caitlin (2021-07-24). "CDC urges labs to use COVID tests that can differentiate from flu". Fox News. Retrieved 2021-07-26.