RhoGEF domain
RhoGEF domain describes two distinct structural domains with guanine nucleotide exchange factor (GEF) activity to regulate small GTPases in the Rho family. Rho small GTPases are inactive when bound to GDP but active when bound to GTP; RhoGEF domains in proteins are able to promote GDP release and GTP binding to activate specific Rho family members, including RhoA, Rac1 and Cdc42.
| DH/PH RhoGEF domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
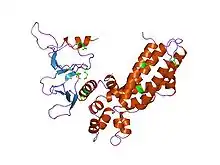 Structure of the RhoGEF domain from the human Son of sevenless protein, an example of a DH/PH domain RhoGEF.[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | RhoGEF | ||||||||||
| Pfam | PF00621 | ||||||||||
| InterPro | IPR035899 IPR000219, IPR035899 | ||||||||||
| SMART | RhoGEF | ||||||||||
| SCOP2 | 1dbh / SCOPe / SUPFAM | ||||||||||
| OPM protein | 1xd4 | ||||||||||
| CDD | cd00160 | ||||||||||
| |||||||||||
| Dedicator of cytokinesis (DOCK) RhoGEF domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbol | RhoGEF | ||||||||||
| Pfam | PF06920 | ||||||||||
| InterPro | IPR010703 IPR026791, IPR010703 | ||||||||||
| SCOP2 | 1wg7 / SCOPe / SUPFAM | ||||||||||
| CDD | cd11684 | ||||||||||
| |||||||||||
The largest class of RhoGEFs is composed of proteins containing the "Dbl-homology" (DH) domain, which almost always is found together with a pleckstrin-homology (PH) domain to form a combined DH/PH domain structure.[2][3]
A distinct class of RhoGEFs is those proteins containing the DOCK/CZH/DHR-2 domain. This structure has no sequence similarity with DBL-homology domains.[4]
Human proteins containing DH/PH RhoGEF domain
ABR; AKAP13/ARHGEF13/Lbc; ALS2; ALS2CL; ARHGEF1/p115-RhoGEF; ARHGEF10; ARHGEF10L; ARHGEF11/PDZ-RhoGEF.; ARHGEF12/LARG; ARHGEF15; ARHGEF16; ARHGEF17; ARHGEF18; ARHGEF19; ARHGEF2; ARHGEF25; ARHGEF26; ARHGEF28; ARHGEF3; ARHGEF33; ARHGEF35; ARHGEF37; ARHGEF38; ARHGEF39; ARHGEF4; ARHGEF40; ARHGEF5; ARHGEF6/alpha-PIX; ARHGEF7/beta-PIX; ARHGEF9; BCR; DNMBP; ECT2; ECT2L; FARP1; FARP2; FGD1; FGD2; FGD3; FGD4; FGD5; FGD6; ITSN1/Intersectin 1; ITSN2/Intersectin 2; KALRN/Kalirin; MCF2; MCF2L; MCF2L2; NET1; NGEF; OBSCN; PLEKHG1; PLEKHG2; PLEKHG3; PLEKHG4; PLEKHG4B; PLEKHG5; PLEKHG6; PREX1; PREX2; RASGRF1; RASGRF2; SPATA13; TIAM1; TIAM2; TRIO; VAV1; VAV2; VAV3.
Human proteins containing DOCK/CZH RhoGEF domain
DOCK1/DOCK180; DOCK2; DOCK3/MOCA; DOCK4; DOCK5; DOCK6/ZIR1; DOCK7/ZIR2; DOCK8/ZIR3; DOCK9/Zizimin1; DOCK10/Zizimin2; DOCK11/Zizimin3
References
- Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J (October 1998). "Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein". Cell. 95 (2): 259–68. doi:10.1016/S0092-8674(00)81756-0. PMID 9790532. S2CID 11868669.
- Fort P, Blangy A (June 2017). "The Evolutionary Landscape of Dbl-Like RhoGEF Families: Adapting Eukaryotic Cells to Environmental Signals". Genome Biology and Evolution. 9 (6): 1471–1486. doi:10.1093/gbe/evx100. PMC 5499878. PMID 28541439.
- Cerione RA, Zheng Y (April 1996). "The Dbl family of oncogenes". Current Opinion in Cell Biology. 8 (2): 216–22. doi:10.1016/s0955-0674(96)80068-8. PMID 8791419.
- Côté JF, Vuori K (December 2002). "Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity". Journal of Cell Science. 115 (Pt 24): 4901–13. doi:10.1242/jcs.00219. PMID 12432077.
Further reading
- Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA (November 1991). "Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product". Nature. 354 (6351): 311–4. doi:10.1038/354311a0. PMID 1956381. S2CID 4240053.
- Tan EC, Leung T, Manser E, Lim L (December 1993). "The human active breakpoint cluster region-related gene encodes a brain protein with homology to guanine nucleotide exchange proteins and GTPase-activating proteins". The Journal of Biological Chemistry. 268 (36): 27291–8. doi:10.1016/S0021-9258(19)74248-3. PMID 8262969.
- Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J (October 1998). "Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein". Cell. 95 (2): 259–68. doi:10.1016/S0092-8674(00)81756-0. PMID 9790532. S2CID 11868669.