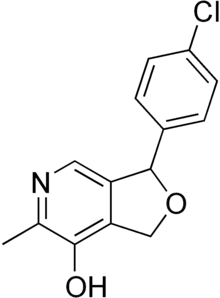
Cicletanine
Cicletanine is a furopyridine low-ceiling diuretic drug, usually used in the treatment of hypertension.[1] The drug is manufactured by Ipsen and marketed by Recordati (in France) under the trade name Tenstaten.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 97.3% |
| Elimination half-life | 7.9 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.158.583 |
| Chemical and physical data | |
| Formula | C14H12ClNO2 |
| Molar mass | 261.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It appears to be more potent in salt-sensitive hypertension.[2]
Mechanism
It can inhibit protein kinase C.[3]
References
- Jean Sassard (1992). Genetic Hypertension. John Libbey Eurotext. ISBN 978-0-86196-313-3.
- Bagrov AY; Dmitrieva RI; Dorofeeva NA; et al. (February 2000). "Cicletanine reverses vasoconstriction induced by the endogenous sodium pump ligand, marinobufagenin, via a protein kinase C dependent mechanism". J. Hypertens. 18 (2): 209–15. doi:10.1097/00004872-200018020-00012. PMID 10694190. S2CID 35374482.
- Fedorova OV, Talan MI, Agalakova NI, Droy-Lefaix MT, Lakatta EG, Bagrov AY (March 2003). "Myocardial PKC beta2 and the sensitivity of Na/K-ATPase to marinobufagenin are reduced by cicletanine in Dahl hypertension". Hypertension. 41 (3): 505–11. doi:10.1161/01.HYP.0000053446.43894.9F. PMID 12623951.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.