Anion-conducting channelrhodopsin
Anion-conducting channelrhodopsins are light-gated ion channels that open in response to light and let negatively charged ions (such as chloride) enter a cell. All channelrhodopsins use retinal as light-sensitive pigment, but they differ in their ion selectivity. Anion-conducting channelrhodopsins are used as tools to manipulate brain activity in mice, fruit flies and other model organisms (Optogenetics). Neurons expressing anion-conducting channelrhodopsins are silenced when illuminated with light, an effect that has been used to investigate information processing in the brain. For example, suppressing dendritic calcium spikes in specific neurons with light reduced the ability of mice to perceive a light touch to a whisker.[2] Studying how the behavior of an animal changes when specific neurons are silenced allows scientists to determine the role of these neurons in the complex circuits controlling behavior.
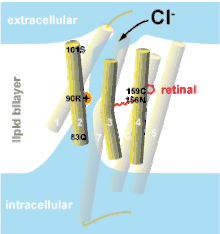
The first anion-conducting channelrhodopsins were engineered from the cation-conducting light-gated channel Channelrhodopsin-2 by removing negatively charged amino acids from the channel pore (Fig. 1).[3] As the main anion of extracellular fluid is chloride (Cl−), anion-conducting channelrhodopsins are also known as “chloride-conducting channelrhodopsins” (ChloCs). Naturally occurring anion-conducting channelrhodopsins (ACRs) were subsequently identified in cryptophyte algae.[4][5][6] The crystal structure of the natural GtACR1 has recently been solved, paving the way for further protein engineering.[7][8]
Variants
| name | species of origin | absorption | reference | properties, applications |
|---|---|---|---|---|
| slowChloC | Chlamydomonas reinhardtii | blue | Wietek et al. 2014[3] | first generation, mixed conductance |
| iC1C2 | Chlamydomonas reinhardtii | blue | Berndt et al. 2014[10] | first generation, mixed conductance |
| iChloC | Chlamydomonas reinhardtii | blue | Wietek et al. 2015[1] | inhibition of perception in mice[2] |
| iC++ | Chlamydomonas reinhardtii | blue | Berndt et al. 2016[11] | inhibition of sleep in mice[12] |
| GtACR1 | Guillardia theta | green | Govorunova et al. 2015[4] | inhibition of behavior in Drosophila[13][14] inhibition of rat heart muscle cells[15] holographic spike suppression in mouse cortex[16] |
| GtACR1(C102A) | Guillardia theta | green on
red off |
Govorunova et al. 2018[6] | bistable |
| GtACR1(R83Q/N239Q) FLASH | Guillardia theta | green on | Kato et al. 2018[7] | very fast closing, large currents
inhibition of swimming in C. elegans, inhibition of spiking in mouse[7] |
| GtACR2 | Guillardia theta | blue | Govorunova et al. 2015[4] | inhibition of behavior in Drosophila[13] inhibition of fear extinction in mice[17] |
| PsACR1 | Proteomonas sulcata | green | Wietek et al. 2016,[18] Govorunova et al. 2016[19] | large currents |
| ZipACR | Proteomonas sulcata | green | Govorunova et al. 2017[5] | very fast |
| RapACR | Rhodomonas salina | green | Govorunova et al. 2018[6] | very fast, large currents |
| SwiChR++ | Chlamydomonas reinhardtii | blue on
red off |
Berndt et al. 2016[11] | bistable |
| Phobos CA | Chlamydomonas reinhardtii | blue on
red off |
Wietek et al. 2017[20] | bistable |
| Aurora | Chlamydomonas reinhardtii | orange-red | Wietek et al. 2017[20] | stop locomotion of Drosophila larvae |
| MerMAIDs | unknown | green | Oppermann et al. 2019[21] | rapidly inactivating |
Applications
Anion-conducting channelrhodopsins (ACRs) have been used as optogenetic tools to inhibit neuronal activation. When expressed in nerve cells, ACRs act as light-gated chloride channels. Their effect on the activity of the neuron is comparable to GABAA receptors, ligand-gated chloride channels found in inhibitory synapses: As the chloride concentration in mature neurons is very low, illumination results in an inward flux of negatively charged ions, clamping the neuron at the chloride reversal potential (- 65 mV). Under these conditions, excitatory synaptic inputs are not able to efficiently depolarize the neuron. This effect is known as shunting inhibition (as opposed to inhibition by hyperpolarization). Illuminating the dendrite prevents the generation of dendritic calcium spikes while illumination of the entire neuron blocks action potential initiation in response to sensory stimulation.[2][1] Axon terminals, however, have a higher chloride concentration and are therefore excited by ACRs.[22] To inhibit neurons with wide-field illumination, it has proven useful to restrict ACRs to the somatic compartment (ST variants).[17][16]
Due to their high light sensitivity, ACRs can be activated with dim light which does not interfere with visual stimulation, even in very small animals like the fruit fly Drosophila.[14] When combined with a red-light sensitive cation-conducting channelrhodopsin, ACRs allow for bidirectional control of neurons: Silencing with blue light, activation with red light ('Bipoles').[23]
Further reading
Neuron Review (2017): Silencing neurons: Tools, Applications, and Experimental Constraints[24]
Research highlight: A better way to turn off neurons[25]
Perspective: Expanding the optogenetics toolkit[26]
Related: Halorhodopsin, a light-driven chloride pump
References
- Wietek, Jonas; Beltramo, Riccardo; Scanziani, Massimo; Hegemann, Peter; Oertner, Thomas G.; Wiegert, J. Simon (2015-10-07). "An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo". Scientific Reports. 5: 14807. doi:10.1038/srep14807. ISSN 2045-2322. PMC 4595828. PMID 26443033.
- Takahashi, Naoya; Oertner, Thomas G.; Hegemann, Peter; Larkum, Matthew E. (2016-12-23). "Active cortical dendrites modulate perception". Science. 354 (6319): 1587–1590. doi:10.1126/science.aah6066. ISSN 0036-8075. PMID 28008068. S2CID 28317052.
- Wietek, Jonas; Wiegert, J. Simon; Adeishvili, Nona; Schneider, Franziska; Watanabe, Hiroshi; Tsunoda, Satoshi P.; Vogt, Arend; Elstner, Marcus; Oertner, Thomas G.; Hegemann, Peter (2014-04-25). "Conversion of Channelrhodopsin into a Light-Gated Chloride Channel". Science. 344 (6182): 409–412. doi:10.1126/science.1249375. ISSN 0036-8075. PMID 24674867. S2CID 206554245.
- Govorunova, Elena G.; Sineshchekov, Oleg A.; Janz, Roger; Liu, Xiaoqin; Spudich, John L. (2015-08-07). "Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics". Science. 349 (6248): 647–650. doi:10.1126/science.aaa7484. ISSN 0036-8075. PMC 4764398. PMID 26113638.
- Govorunova, Elena G.; Sineshchekov, Oleg A.; Rodarte, Elsa M.; Janz, Roger; Morelle, Olivier; Melkonian, Michael; Wong, Gane K.-S.; Spudich, John L. (2017-03-03). "The Expanding Family of Natural Anion Channelrhodopsins Reveals Large Variations in Kinetics, Conductance, and Spectral Sensitivity". Scientific Reports. 7: 43358. doi:10.1038/srep43358. ISSN 2045-2322. PMC 5335703. PMID 28256618.
- Govorunova, Elena G.; Sineshchekov, Oleg A.; Hemmati, Raheleh; Janz, Roger; Morelle, Olivier; Melkonian, Michael; Wong, Gane K.-S.; Spudich, John L. (2018-05-01). "Extending the Time Domain of Neuronal Silencing with Cryptophyte Anion Channelrhodopsins". eNeuro. 5 (3): ENEURO.0174–18.2018. doi:10.1523/ENEURO.0174-18.2018. ISSN 2373-2822. PMC 6051594. PMID 30027111.
- Kato, Hideaki E.; Kim, Yoon Seok; Paggi, Joseph M.; Evans, Kathryn E.; Allen, William E.; Richardson, Claire; Inoue, Keiichi; Ito, Shota; Ramakrishnan, Charu (2018-08-29). "Structural mechanisms of selectivity and gating in anion channelrhodopsins". Nature. 561 (7723): 349–354. doi:10.1038/s41586-018-0504-5. ISSN 0028-0836. PMC 6317992. PMID 30158697.
- Kim, Yoon Seok; Kato, Hideaki E.; Yamashita, Keitaro; Ito, Shota; Inoue, Keiichi; Ramakrishnan, Charu; Fenno, Lief E.; Evans, Kathryn E.; Paggi, Joseph M. (2018-08-29). "Crystal structure of the natural anion-conducting channelrhodopsin GtACR1". Nature. 561 (7723): 343–348. doi:10.1038/s41586-018-0511-6. ISSN 0028-0836. PMC 6340299. PMID 30158696.
- Postic, Guillaume; Ghouzam, Yassine; Guiraud, Vincent; Gelly, Jean-Christophe (2016). "Membrane positioning for high- and low-resolution protein structures through a binary classification approach". Protein Engineering, Design and Selection. 29 (3): 87–91. doi:10.1093/protein/gzv063. PMID 26685702.
- Berndt, Andre; Lee, Soo Yeun; Ramakrishnan, Charu; Deisseroth, Karl (2014-04-25). "Structure-Guided Transformation of Channelrhodopsin into a Light-Activated Chloride Channel". Science. 344 (6182): 420–424. doi:10.1126/science.1252367. ISSN 0036-8075. PMC 4096039. PMID 24763591.
- Berndt, Andre; Lee, Soo Yeun; Wietek, Jonas; Ramakrishnan, Charu; Steinberg, Elizabeth E.; Rashid, Asim J.; Kim, Hoseok; Park, Sungmo; Santoro, Adam (2016-01-26). "Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity". Proceedings of the National Academy of Sciences. 113 (4): 822–829. doi:10.1073/pnas.1523341113. ISSN 0027-8424. PMC 4743797. PMID 26699459.
- Chung, Shinjae; Weber, Franz; Zhong, Peng; Tan, Chan Lek; Nguyen, Thuc Nghi; Beier, Kevin T.; Hörmann, Nikolai; Chang, Wei-Cheng; Zhang, Zhe (2017). "Identification of preoptic sleep neurons using retrograde labelling and gene profiling". Nature. 545 (7655): 477–481. doi:10.1038/nature22350. PMC 5554302. PMID 28514446.
- Mohammad, Farhan; Stewart, James C; Ott, Stanislav; Chlebikova, Katarina; Chua, Jia Yi; Koh, Tong-Wey; Ho, Joses; Claridge-Chang, Adam (2017). "Optogenetic inhibition of behavior with anion channelrhodopsins". Nature Methods. 14 (3): 271–274. doi:10.1038/nmeth.4148. PMID 28114289. S2CID 4133602.
- Mauss, Alex S.; Busch, Christian; Borst, Alexander (2017-10-23). "Optogenetic Neuronal Silencing in Drosophila during Visual Processing". Scientific Reports. 7 (1): 13823. doi:10.1038/s41598-017-14076-7. ISSN 2045-2322. PMC 5653863. PMID 29061981.
- Govorunova, Elena G.; Cunha, Shane R.; Sineshchekov, Oleg A.; Spudich, John L. (2016-09-15). "Anion channelrhodopsins for inhibitory cardiac optogenetics". Scientific Reports. 6 (1): 33530. doi:10.1038/srep33530. ISSN 2045-2322. PMC 5024162. PMID 27628215.
- Mardinly, Alan R.; Oldenburg, Ian Antón; Pégard, Nicolas C.; Sridharan, Savitha; Lyall, Evan H.; Chesnov, Kirill; Brohawn, Stephen G.; Waller, Laura; Adesnik, Hillel (2018-04-30). "Precise multimodal optical control of neural ensemble activity". Nature Neuroscience. 21 (6): 881–893. doi:10.1038/s41593-018-0139-8. ISSN 1097-6256. PMC 5970968. PMID 29713079.
- Mahn, Mathias; Gibor, Lihi; Malina, Katayun Cohen-Kashi; Patil, Pritish; Printz, Yoav; Oring, Shir; Levy, Rivka; Lampl, Ilan; Yizhar, Ofer (2018). "High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins". Nature Communications. 9 (1): 4125. doi:10.1038/s41467-018-06511-8. PMC 6175909. PMID 30297821.
- Wietek, Jonas; Broser, Matthias; Krause, Benjamin S.; Hegemann, Peter (2016-02-19). "Identification of a Natural Green Light Absorbing Chloride Conducting Channelrhodopsin from Proteomonas sulcata". Journal of Biological Chemistry. 291 (8): 4121–4127. doi:10.1074/jbc.M115.699637. ISSN 0021-9258. PMC 4759187. PMID 26740624.
- Govorunova, Elena G.; Sineschekov, Oleg A.; Spudich, John L. (2016-02-01). "Proteomonas sulcata ACR1: A Fast Anion Channelrhodopsin". Photochemistry and Photobiology. 92 (2): 257–263. doi:10.1111/php.12558. PMC 4914479. PMID 26686819.
- Wietek, Jonas; Rodriguez-Rozada, Silvia; Tutas, Janine; Tenedini, Federico; Grimm, Christiane; Oertner, Thomas G.; Soba, Peter; Hegemann, Peter; Wiegert, J. Simon (Nov 2017). "Anion-conducting channelrhodopsins with tuned spectra and modified kinetics engineered for optogenetic manipulation of behavior". Scientific Reports. 7 (1): 14957. doi:10.1038/s41598-017-14330-y. ISSN 2045-2322. PMC 5668261. PMID 29097684.
- Oppermann, Johannes; Fischer, Paul; Silapetere, Arita; Liepe, Bernhard; Rodriguez-Rozada, Silvia; Flores-Uribe, José; Peter, Enrico; Keidel, Anke; Vierock, Johannes; Kaufmann, Joel; Broser, Matthias (2019-07-25). "MerMAIDs: a family of metagenomically discovered marine anion-conducting and intensely desensitizing channelrhodopsins". Nature Communications. 10 (1): 3315. doi:10.1038/s41467-019-11322-6. ISSN 2041-1723. PMC 6658528. PMID 31346176.
- Mahn, Mathias; Prigge, Matthias; Ron, Shiri; Levy, Rivka; Yizhar, Ofer (2016). "Biophysical constraints of optogenetic inhibition at presynaptic terminals". Nature Neuroscience. 19 (4): 554–556. doi:10.1038/nn.4266. PMC 4926958. PMID 26950004.
- Vierock, Johannes; Rodriguez-Rozada, Silvia; Dieter, Alexander; Pieper, Florian; Sims, Ruth; Tenedini, Federico; Bergs, Amelie C. F.; Bendifallah, Imane; Zhou, Fangmin; Zeitzschel, Nadja; Ahlbeck, Joachim (2021-07-26). "BiPOLES is an optogenetic tool developed for bidirectional dual-color control of neurons". Nature Communications. 12 (1): 4527. doi:10.1038/s41467-021-24759-5. ISSN 2041-1723. PMC 8313717. PMID 34312384.
- Wiegert, J. Simon; Mahn, Mathias; Prigge, Matthias; Printz, Yoav; Yizhar, Ofer (2017). "Silencing Neurons: Tools, Applications, and Experimental Constraints". Neuron. 95 (3): 504–529. doi:10.1016/j.neuron.2017.06.050. PMC 5830081. PMID 28772120.
- Evanko, Daniel (2014). "Neuroscience: A better way to turn off neurons". Nature Methods. 11 (6): 608. doi:10.1038/nmeth.2988. S2CID 1699434.
- Berndt, Andre; Deisseroth, Karl (2015-08-07). "Expanding the optogenetics toolkit". Science. 349 (6248): 590–591. doi:10.1126/science.aac7889. ISSN 0036-8075. PMC 4776750. PMID 26250674.