Ammonium acetate
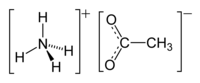
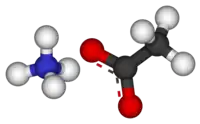
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially.[5]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ammonium ethanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.149 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H7NO2 | |
| Molar mass | 77.083 g·mol−1 |
| Appearance | White solid crystals, deliquescent |
| Odor | Slightly acetic acid like |
| Density | 1.17 g/cm3 (20 °C)[1] 1.073 g/cm3 (25 °C) |
| Melting point | 113 °C (235 °F; 386 K)[2] |
| 102 g/100 mL (0 °C) 148 g/100 mL (4 °C)[1] 143 g/100 mL (20 °C) 533 g/100 mL (80 °C) | |
| Solubility | Soluble in alcohol, SO2, acetone, liquid ammonia[3] |
| Solubility in methanol | 7.89 g/100 mL (15 °C)[4][1] 131.24 g/100 g (94.2 °C)[3] |
| Solubility in dimethylformamide | 0.1 g/100 g[3] |
| Acidity (pKa) | 9.9 |
| Basicity (pKb) | 33 |
| -41.1·10−6 cm3/mol | |
| Viscosity | 21 |
| Structure | |
| Orthorhombic | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−615 kJ/mol[3] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling:[4] | |
 | |
| Warning | |
| H303, H316, H320, H333 | |
| P281, P335 | |
| NFPA 704 (fire diamond) | |
| Flash point | 136 °C (277 °F; 409 K)[4] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
386 mg/kg (mice, intravenous)[3] |
| Safety data sheet (SDS) | JT Baker |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
It is the main precursor to acetamide:[7]
- NH4CH3CO2 → CH3C(O)NH2 + H2O
It is also used as a diuretic.[5]
Buffer
As the salt of a weak acid and a weak base, ammonium acetate is often used with acetic acid to create a buffer solution. Ammonium acetate is volatile at low pressures. Because of this, it has been used to replace cell buffers that contain non-volatile salts in preparing samples for mass spectrometry.[8] It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate.
When dissolving ammonium acetate in pure water, the resulting solution typically has a pH of 7, because the equal amounts of acetate and ammonium neutralize each other. However, ammonium acetate is a dual component buffer system, which buffers around pH 4.75 ± 1 (acetate) and pH 9.25 ± 1 (ammonium), but it has no significant buffer capacity at pH 7, contrary to common misconception.[9]
Other
- a biodegradable de-icing agent.
- a catalyst in the Knoevenagel condensation and as a source of ammonia in the Borch reaction in organic synthesis.
- a protein precipitating reagent in dialysis to remove contaminants via diffusion.
- a reagent in agricultural chemistry for determination of soil CEC (cation exchange capacity) and determination of available potassium in soil wherein the ammonium ion acts as a replacement cation for potassium.
- part of Calley's method for lead artifact conservation
Food additive
Ammonium acetate is also used as a food additive as an acidity regulator; INS number 264. It is approved for usage in Australia and New Zealand.[10]
Production
Ammonium acetate is produced by the neutralization of acetic acid with ammonium carbonate or by saturating glacial acetic acid with ammonia.[11] Obtaining crystalline ammonium acetate is difficult on account of its hygroscopic nature.
References
- Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. ISBN 0-07-049439-8.
- Davidson, Arthur W.; McAllister, Walter H. (1930). "Solutions of Salts in Pure Acetic Acid. Ii. Solubilities of Acetates1". Journal of the American Chemical Society. 52 (2): 507–519. doi:10.1021/ja01365a010. ISSN 0002-7863.
- "Ammonium acetate".
- "Safety Data Sheet of Ammonium Acetate" (PDF). tedia.com. Tedia Company Inc. 2011-08-12. Retrieved 2014-06-10.
- Hosea Cheung; Robin S. Tanke; G. Paul Torrence. "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.pub2.
- "Spirit of Mindererus". TheFreeDictionary.com. Retrieved 2023-06-07.
- Coleman, G. H.; Alvarado, A. M. (1923). "Acetamide". Organic Syntheses. 3: 3.; Collective Volume, vol. 1, p. 3
- Berman, Elena S. F.; Fortson, Susan L.; Checchi, Kyle D.; Wu, Ligang; Felton, James S.; Kuang Jen, J. Wu; Kulp, Kristen S. (2008). "Preparation of single cells for imaging/profiling mass spectrometry". J Am Soc Mass Spectrom. 19 (8): 1230–1236. doi:10.1016/j.jasms.2008.05.006. PMID 18565760.
- Konermann, Lars (2017). "Addressing a Common Misconception: Ammonium Acetate as Neutral pH "Buffer" for Native Electrospray Mass Spectrometry". American Society for Mass Spectrometry. 28 (9): 1827–1835. Bibcode:2017JASMS..28.1827K. doi:10.1007/s13361-017-1739-3. PMID 28710594. S2CID 25294943. Retrieved 25 October 2022.
- Australia New Zealand Food Standards Code "Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- Brannt, William (1914). A practical treatise on the manufacture of vinegar. Lancaster, PA: Henry Carey Baird & Co. pp. 316–317.
