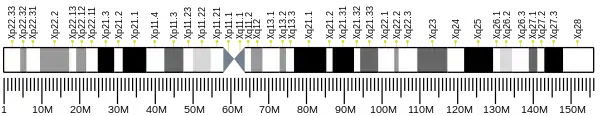
ALAS2
Delta-aminolevulinate synthase 2 also known as ALAS2 is a protein that in humans is encoded by the ALAS2 gene.[5][6][7] ALAS2 is an aminolevulinic acid synthase.
| ALAS2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ALAS2, ALAS-E, ALASE, ANH1, ASB, XLDPP, XLEPP, XLSA, SIDBA1, 5'-aminolevulinate synthase 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 301300 MGI: 87990 HomoloGene: 17 GeneCards: ALAS2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
The product of this gene specifies an erythroid-specific mitochondrially located enzyme. The encoded protein catalyzes the first step in the heme biosynthetic pathway. Defects in this gene cause X-linked pyridoxine-responsive sideroblastic anemia. Alternatively spliced transcript variants encoding different isoforms have been identified.[7]
Its gene contains an IRE in its 5'-UTR region on which an IRP binds if the iron level is too low, thus inhibiting its translation.
References
- GRCh38: Ensembl release 89: ENSG00000158578 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000025270 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Bishop DF, Henderson AS, Astrin KH (Jun 1990). "Human delta-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the X chromosome". Genomics. 7 (2): 207–14. doi:10.1016/0888-7543(90)90542-3. PMID 2347585.
- Cotter PD, Willard HF, Gorski JL, Bishop DF (May 1992). "Assignment of human erythroid delta-aminolevulinate synthase (ALAS2) to a distal subregion of band Xp11.21 by PCR analysis of somatic cell hybrids containing X; autosome translocations" (PDF). Genomics. 13 (1): 211–2. doi:10.1016/0888-7543(92)90223-F. hdl:2027.42/30074. PMID 1577484.
- "Entrez Gene: Delta-aminolevulinate synthase 2".
Further reading
- Han L, Zhong Y, Huang B, Han L, Pan L, Xu X, Wang X, Huang B, Lu J (2008). "Sodium butyrate activates erythroid-specific 5-aminolevulinate synthase gene through Sp1 elements at its promoter". Blood Cells, Molecules & Diseases. 41 (2): 148–53. doi:10.1016/j.bcmd.2008.04.002. PMID 18555711.
- Kaneko K, Furuyama K, Aburatani H, Shibahara S (Mar 2009). "Hypoxia induces erythroid-specific 5-aminolevulinate synthase expression in human erythroid cells through transforming growth factor-beta signaling". The FEBS Journal. 276 (5): 1370–82. doi:10.1111/j.1742-4658.2009.06878.x. PMID 19187226.
- Cox TC, Sadlon TJ, Schwarz QP, Matthews CS, Wise PD, Cox LL, Bottomley SS, May BK (Feb 2004). "The major splice variant of human 5-aminolevulinate synthase-2 contributes significantly to erythroid heme biosynthesis". The International Journal of Biochemistry & Cell Biology. 36 (2): 281–95. doi:10.1016/S1357-2725(03)00246-2. PMID 14643893.
- Harigae H, Furuyama K, Kudo K, Hayashi N, Yamamoto M, Sassa S, Sasaki T (Oct 1999). "A novel mutation of the erythroid-specific gamma-Aminolevulinate synthase gene in a patient with non-inherited pyridoxine-responsive sideroblastic anemia". American Journal of Hematology. 62 (2): 112–4. doi:10.1002/(SICI)1096-8652(199910)62:2<112::AID-AJH9>3.0.CO;2-L. PMID 10577279.
- Hurford MT, Marshall-Taylor C, Vicki SL, Zhou JZ, Silverman LM, Rezuke WN, Altman A, Tsongalis GJ (Jul 2002). "A novel mutation in exon 5 of the ALAS2 gene results in X-linked sideroblastic anemia". Clinica Chimica Acta; International Journal of Clinical Chemistry. 321 (1–2): 49–53. doi:10.1016/S0009-8981(02)00095-5. PMID 12031592.
- Bekri S, May A, Cotter PD, Al-Sabah AI, Guo X, Masters GS, Bishop DF (Jul 2003). "A promoter mutation in the erythroid-specific 5-aminolevulinate synthase (ALAS2) gene causes X-linked sideroblastic anemia". Blood. 102 (2): 698–704. doi:10.1182/blood-2002-06-1623. PMID 12663458.
- Astner I, Schulze JO, van den Heuvel J, Jahn D, Schubert WD, Heinz DW (Sep 2005). "Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans". The EMBO Journal. 24 (18): 3166–77. doi:10.1038/sj.emboj.7600792. PMC 1224682. PMID 16121195.
- Cazzola M, May A, Bergamaschi G, Cerani P, Ferrillo S, Bishop DF (Dec 2002). "Absent phenotypic expression of X-linked sideroblastic anemia in one of 2 brothers with a novel ALAS2 mutation". Blood. 100 (12): 4236–8. doi:10.1182/blood-2002-03-0685. PMID 12393718.
- Sussman NL, Lee PL, Dries AM, Schwartz MR, Barton JC (2008). "Multi-organ iron overload in an African-American man with ALAS2 R452S and SLC40A1 R561G". Acta Haematologica. 120 (3): 168–73. doi:10.1159/000181183. PMID 19066423. S2CID 10636344.
- Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M, Corrigall AV, Meissner PN, Hift RJ, Marsden JT, Ma Y, Mieli-Vergani G, Deybach JC, Puy H (Sep 2008). "C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload". American Journal of Human Genetics. 83 (3): 408–14. doi:10.1016/j.ajhg.2008.08.003. PMC 2556430. PMID 18760763.
- Furuyama K, Sassa S (Mar 2000). "Interaction between succinyl CoA synthetase and the heme-biosynthetic enzyme ALAS-E is disrupted in sideroblastic anemia". The Journal of Clinical Investigation. 105 (6): 757–64. doi:10.1172/JCI6816. PMC 377455. PMID 10727444.
- Suzuki Y, Yamashita R, Shirota M, Sakakibara Y, Chiba J, Mizushima-Sugano J, Nakai K, Sugano S (Sep 2004). "Sequence comparison of human and mouse genes reveals a homologous block structure in the promoter regions". Genome Research. 14 (9): 1711–8. doi:10.1101/gr.2435604. PMC 515316. PMID 15342556.
- Lee PL, Barton JC, Rao SV, Acton RT, Adler BK, Beutler E (2006). "Three kinships with ALAS2 P520L (c. 1559 C --> T) mutation, two in association with severe iron overload, and one with sideroblastic anemia and severe iron overload". Blood Cells, Molecules & Diseases. 36 (2): 292–7. doi:10.1016/j.bcmd.2005.12.004. PMID 16446107.
- Bergmann AK, Campagna DR, McLoughlin EM, Agarwal S, Fleming MD, Bottomley SS, Neufeld EJ (Feb 2010). "Systematic molecular genetic analysis of congenital sideroblastic anemia: evidence for genetic heterogeneity and identification of novel mutations". Pediatric Blood & Cancer. 54 (2): 273–8. doi:10.1002/pbc.22244. PMC 2843911. PMID 19731322.
- Rabstein S, Unfried K, Ranft U, Illig T, Kolz M, Mambetova C, Vlad M, Roman C, Weiss T, Becker D, Brüning T, Pesch B (2008). "Lack of association of delta-aminolevulinate dehydratase polymorphisms with blood lead levels and hemoglobin in Romanian women from a lead-contaminated region". Journal of Toxicology and Environmental Health. Part A. 71 (11–12): 716–24. doi:10.1080/15287390801985190. PMID 18569569. S2CID 20337081.
- Abu-Farha M, Niles J, Willmore WG (Oct 2005). "Erythroid-specific 5-aminolevulinate synthase protein is stabilized by low oxygen and proteasomal inhibition". Biochemistry and Cell Biology. 83 (5): 620–30. doi:10.1139/o05-045. PMID 16234850.
- Nachman MW, D'Agostino SL, Tillquist CR, Mobasher Z, Hammer MF (May 2004). "Nucleotide variation at Msn and Alas2, two genes flanking the centromere of the X chromosome in humans". Genetics. 167 (1): 423–37. doi:10.1534/genetics.167.1.423. PMC 1470878. PMID 15166166.
External links
- Human ALAS2 genome location and ALAS2 gene details page in the UCSC Genome Browser.
- GeneReviews/NCBI/NIH/UW entry on X-Linked Sideroblastic Anemia and Ataxia
This article incorporates text from the United States National Library of Medicine, which is in the public domain.