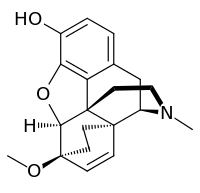
6,14-Endoethenotetrahydrooripavine
6,14-Endoethenotetrahydrooripavine is the central nucleus, or backbone, of a class of morphinan opioids known as the Bentley compounds and may be considered their "privileged scaffold".[1][2] These include but are not limited to etorphine and buprenorphine. They usually have thebaine or oripavine as their precursor in their syntheses (and are thus termed "thevinols" and "orvinols", respectively).
 | |
| Names | |
|---|---|
| IUPAC name
(4R,7S,7aR,12bR)-7-Methoxy-3-methyl-2,3,4,4a,7,7a-hexahydro-1H-4al5-4a,7-ethano-4,12-methanobenzofuro[3,2-e]isoquinolin-9-ol | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C20H23NO3 | |
| Molar mass | 325.408 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Bentley, Kenneth W.; Hardy, Denis G. (June 1967). "Novel analgesics and molecular rearrangements in the morphine-thebaine group. III. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allylnormorphine and -norcodeine". Journal of the American Chemical Society. 89 (13): 3281–3292. doi:10.1021/ja00989a032. ISSN 0002-7863. PMID 6042764.
- PubChem. "6,14-Endoethenotetrahydrooripavine". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-11-21.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.