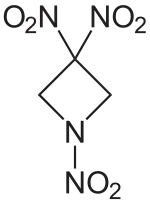
1,3,3-Trinitroazetidine
1,3,3-Trinitroazetidine (TNAZ) is a highly energetic heterocyclic compound that has been considered as a potential replacement for TNT because of its low melting point (101 °C) and good thermal stability (up to 240 °C). TNAZ was first synthesized by Archibald et al. in 1990.[3] Several synthesis routes are known, and bulk production of several hundred kilogram batches has been demonstrated at Los Alamos National Laboratory.[4][1][5]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,3-Trinitroazetidine | |
| Other names
TNAZ | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4N4O6 | |
| Molar mass | 192.087 g·mol−1 |
| Appearance | Pale yellow crystals |
| Density | 1.84 g/cm3 |
| Melting point | 101[1] °C (214 °F; 374 K) |
| Boiling point | 252[1] °C (486 °F; 525 K) |
| Structure | |
| Orthorhombic | |
| Explosive data | |
| Detonation velocity | 9597 m/s[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties
The compound crystallizes in an orthorhombic lattice with the space group Pbca. Thermolysis occurs starting around 240 °C - 250 °C with decomposition products that include nitrogen dioxide, nitric oxide, nitrous acid, carbon dioxide, and formaldehyde. It has a heat of decomposition of 6343 kJ/kg, and a detonation pressure of 36.4 GPa.[6]
References
- Viswanath, Dabir S.; Ghosh, Tushar K.; Boddu, Veera M. (2018). "1,3,3-Trinitroazetidine (TNAZ)". Emerging Energetic Materials: Synthesis, Physicochemical, and Detonation Properties. pp. 293–307. doi:10.1007/978-94-024-1201-7_11. ISBN 978-94-024-1199-7.
- Simpson, R.L.; Garza, R.G.; Foltz, M.F.; Ornellas, D.L.; Utriew, P.A. (14 December 1994). Characterization of TNAZ (PDF) (Technical report). Office of Scientific and Technical Information (OSTI). doi:10.2172/71573. OSTI 71573.
- Archibald, T. G; Gilardi, Richard; Baum, K; George, Clifford (1990). "Synthesis and x-ray crystal structure of 1,3,3-trinitroazetidine". The Journal of Organic Chemistry. 55 (9): 2920–2924. doi:10.1021/jo00296a066.
- Coburn, Michael D.; Hiskey, Michael A.; Archibald, Thomas G. (January 1998). "Scale-up and waste-minimization of the Los Alamos process for 1,3,3-trinitroazetidine (TNAZ)". Waste Management. 17 (2–3): 143–146. Bibcode:1998WaMan..17..143C. doi:10.1016/S0956-053X(97)10013-7.
- Jalový, Zdenek; Zeman, Svatopluk; Suceska, Muhamed; Vávra, Pave; Dudek, Kamil; Rajic, Masa (1 June 2001). "1,3,3-trinitroazetidine (TNAZ). Part I. Syntheses and properties". Journal of Energetic Materials. 19 (2): 219–239. Bibcode:2001JEnM...19..219J. doi:10.1080/07370650108216127. ISSN 0737-0652. S2CID 98003295.
- Axenrod, Theodore; Watnick, Clara; Yazdekhasti, Hamid; Dave, Paritosh R (1993). "Synthesis of 1,3,3-trinitroazetidine". Tetrahedron Letters. 34 (42): 6677–6680. doi:10.1016/S0040-4039(00)61673-8.