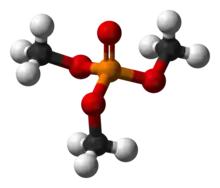
Trimethyl phosphate
Trimethyl phosphate is the trimethyl ester of phosphoric acid. It is a colourless, nonvolatile liquid. It has some specialized uses in the production of other compounds.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Trimethyl phosphate | |
| Other names
Phosphoric acid trimethyl ester Methyl phosphate, tribasic Trimethoxyphosphine oxide Trimethyl orthophosphate fraction | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TMP |
| 1071731 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.405 |
| 49926 | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (CH3O)3PO | |
| Molar mass | 140.08 |
| Appearance | Colorless liquid |
| Melting point | −46 °C (−51 °F; 227 K) |
| Boiling point | 197 °C (387 °F; 470 K) |
| good | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H302, H315, H318, H319, H340, H350, H351, H361, H373 | |
| P201, P202, P260, P264, P270, P280, P281, P301+P312, P302+P352, P305+P351+P338, P308+P313, P310, P314, P321, P330, P332+P313, P337+P313, P362, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
Trimethyl phosphate is prepared by treating phosphorus oxychloride with methanol in the presence of an amine base:
- POCl3 + 3 CH3OH + 3 R3N → PO(OCH3)3 + 3 R3NH+Cl−
It is a tetrahedral molecule that is a weakly polar solvent.
Applications
Trimethyl phosphate is a mild methylating agent, useful for dimethylation of anilines and related heterocyclic compounds.[2] The method is complementary to the traditional Eschweiler-Clarke reaction in cases where formaldehyde engages in side reactions.
Trimethyl phosphate is used as a solvent for aromatic halogenations and nitrations as required for the preparation of pesticides and pharmaceuticals.
Other applications
It is used as a color inhibitor for fibers (e.g. polyester) and other polymers. Trimethyl phosphate is used as a simulant for chemical weapon nerve agents.
Safety considerations
With an LD50 of 2g/kg for rats, trimethylphosphate is expected to have low acute toxicity.[3]
References
- D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam 1995. ISBN 0-444-89307-5.
- William A. Sheppard (1973). "m-Trifluoromethyl-N,N-dimethylaniline". Organic Syntheses.; Collective Volume, vol. 5, p. 1085
- J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a19_545.pub2
