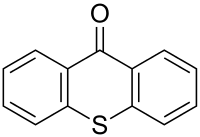
Thioxanthone
Thioxanthone is a heterocyclic compound that is a sulfur analog of xanthone.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Thioxanthen-9-one | |
| Other names
Thioxanthenone; 9-Oxothioxanthene; Thioxanthen-9-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.046 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H8OS | |
| Molar mass | 212.27 g·mol−1 |
| Appearance | Pale yellow solid[1] |
| Melting point | 211 °C (412 °F; 484 K)[2] |
| Boiling point | 273 °C (523 °F; 546 K)[2] (940 hPa) |
| Nearly insoluble | |
| Solubility in sulfuric acid | Soluble[2] |
| -130·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Thioxanthone can be prepared by the reaction of diphenyl sulfide with phosgene in the presence of catalytic aluminium chloride.[2] This synthesis can be seen as a special case of the Friedel-Crafts acylation. The reduction product is thioxanthene.
Thioxanthone dissolves in concentrated sulfuric acid to give a yellow colored liquid with intense green fluorescence. A mixture of the thioxanthone derivatives of 2- and 4-isopropylthioxanthone (ITX) is used in the printing industry. Pharmaceutical drugs that are derivatives of thioxanthone include hycanthone and lucanthone.
References
- Thioxanthone at Sigma-Aldrich
- Merck Index, 14th Edition, 1610
External links
 Media related to thioxanthones at Wikimedia Commons
Media related to thioxanthones at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.