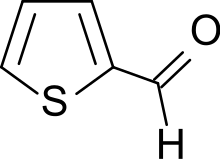
Thiophene-2-carboxaldehyde
Thiophene-2-carboxaldehyde is an organosulfur compound with the formula C4H3SCHO. It is one of two isomeric thiophenecarboxaldehydes. It is a colorless liquid that often appears amber after storage. It is versatile precursor to many drugs including eprosartan, Azosemide, and Teniposide.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Thiophene-2-carbaldehyde | |
| Other names
2-formylthiophene, thiophene-2-aldehyde, T2A, 2-thiophenecarboxaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.391 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H4OS | |
| Molar mass | 112.15 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.2 g/mL |
| Boiling point | 198 °C (388 °F; 471 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H315, H317, H319, H335 | |
| P261, P264, P270, P271, P272, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
It can be prepared from thiophene by the Vilsmeier reaction.[1] Alternatively, it is prepared from chloromethylation of thiophene.[2]
References
- Jonathan Swanston (2006). "Thiophene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2.
- Kenneth B. Wiberg. "2-Thiophenealdehyde". Org. Synth. 3: 811. doi:10.15227/orgsyn.000.0005.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.