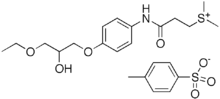
Suplatast tosilate
Suplatast tosilate (INN) is a Th2 cytokine inhibitor[1] which is used as an antihistamine. It has been suggested and applied in the treatment of Kimura's disease a few times.[2][3]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.132 |
| Chemical and physical data | |
| Formula | C23H33NO7S2 |
| Molar mass | 499.64 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Tamaoki J, Kondo M, Sakai N, et al. (July 2000). "Effect of suplatast tosilate, a Th2 cytokine inhibitor, on steroid-dependent asthma: a double-blind randomised study. Tokyo Joshi-Idai Asthma Research Group". Lancet. 356 (9226): 273–8. doi:10.1016/S0140-6736(00)02501-0. PMID 11071181. S2CID 25482487.
- Ueda T, Arai S, Amoh Y, Katsuoka K (2011). "Kimura's disease treated with suplatast tosilate and loratadine". European Journal of Dermatology. 21 (6): 1020–1. doi:10.1684/ejd.2011.1539. PMID 21914581.
- Tsukagoshi H, Nagashima M, Horie T, et al. (December 1998). "Kimura's disease associated with bronchial asthma presenting eosinophilia and hyperimmunoglobulinemia E which were attenuated by suplatast tosilate (IPD-1151T)". Internal Medicine. 37 (12): 1064–7. doi:10.2169/internalmedicine.37.1064. PMID 9932643.
Further reading
- Taniguchi H, Togawa M, Ohwada K, et al. (December 1996). "Suplatast tosilate, a new type of antiallergic agent, prevents the expression of airway hyperresponsiveness in guinea pigs". European Journal of Pharmacology. 318 (2–3): 447–54. doi:10.1016/S0014-2999(96)00810-2. PMID 9016937.
- Sano Y (December 1996). "[Anti-inflammatory drugs for the treatment of bronchial hyperresponsiveness]". Nihon Kyōbu Shikkan Gakkai Zasshi (in Japanese). 34 Suppl: 48–53. PMID 9216184.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.