Phycotoxin
Phycotoxins (from Ancient Greek φῦκος (phûkos) 'seaweed', and τοξικόν (toxikón) 'poison, toxin') are complex allelopathic chemicals produced by eukaryotic and prokaryotic algal secondary metabolic pathways. More simply, these are toxic chemicals synthesized by photosynthetic organisms. These metabolites are (in most cases) not harmful to the producer but may be toxic to either one or many members of the marine food web. This page focuses on phycotoxins produced by marine microalgae; however, freshwater algae and macroalgae are known phycotoxin producers and may exhibit analogous ecological dynamics. In the pelagic marine food web, phytoplankton are subjected to grazing by macro- and micro-zooplankton as well as competition for nutrients with other phytoplankton species. Marine bacteria try to obtain a share of organic carbon by maintaining symbiotic, parasitic, commensal, or predatory interactions with phytoplankton. Other bacteria will degrade dead phytoplankton or consume organic carbon released by viral lysis. The production of toxins is one strategy that phytoplankton use to deal with this broad range of predators, competitors, and parasites. Smetacek suggested that "planktonic evolution is ruled by protection and not competition. The many shapes of plankton reflect defense responses to specific attack systems".[1] Indeed, phytoplankton retain an abundance of mechanical and chemical defense mechanisms including cell walls, spines, chain/colony formation, and toxic chemical production. These morphological and physiological features have been cited as evidence for strong predatory pressure in the marine environment.[2] However, the importance of competition is also demonstrated by the production of phycotoxins that negatively impact other phytoplankton species. Flagellates (especially dinoflagellates) are the principle producers of phycotoxins; however, there are known toxigenic diatoms, cyanobacteria, prymnesiophytes, and raphidophytes.[3] Because many of these allelochemicals are large and energetically expensive to produce, they are synthesized in small quantities. However, phycotoxins are known to accumulate in other organisms and can reach high concentrations during algal blooms. Additionally, as biologically active metabolites, phycotoxins may produce ecological effects at low concentrations. These effects may be subtle, but have the potential to impact the biogeographic distributions of phytoplankton and bloom dynamics.
Potential ecological effects
Anti-grazing effects
Phycotoxins may prevent grazing by several mechanisms: grazer death, infertility, or deterrence. Some evidence of anti-grazing effects:
- Teegarden[4] found that three different species of copepods were able to distinguish between a saxitoxin-producing Alexandrium sp. and morphologically similar non-toxigenic Alexandrium sp. by chemosensory means. These three different copepod species grazed predominantly on the non-toxigenic Alexandrium spp. and avoided the saxitoxin-producer. However, the effect of saxitoxin deterrence varied per copepod species. This implies that saxitoxin producing Alexandrium sp. have an advantage over non-toxigenic dinoflagellates.
- Miralto et al.[5] reported a low hatching success of eggs laid by copepods that fed on diatoms containing polyunsaturated aldehydes. When ingested by copepods, these aldehydes appear to arrest embryonic development. This has the potential to decrease the future population of copepods and promote the survival of copepods which do not eat as many diatoms.
Anti-microbial effects
Phycotoxins production may be useful to ward off parasitic or algicidal heterotrophic bacteria. Some evidence of anti-microbial effects:
- Bates et al.[6] was able to enhance domoic acid production in Pseudo-nitzschia multseries with the re-introduction of bacteria. Additionally, P. multiseries cultures which were completely axenic (bacteria-free), produce less domoic acid than P. multiseries cultures which have contained bacteria for several generations.
- Sieburth[7] found acrylic acid inhibited gut microflora in penguins. High concentrations of acrylic acid were ingested by penguins via their euphasid diet, which had been feeding on Phaeocystis. The antimicrobial effect of acrylic acid was verified by Slezak et al.[8] who concluded that acrylic acid will inhibit bacterial production in situations where phytoplankton form aggregates (i.e. marine snow or Phaeocystis blooms). However, acrylic acid production may also serve to keep bacteria away from the phytoplankton in more dilute concentrations.
Competitive effects
Since many different species of phytoplankton compete for a limited number of nutrients (see Paradox of the Plankton), it's possible that phycotoxin production is used as a method to either kill competitors or to keep other phytoplankton out of the producer's nutrients space. Some evidence of competitive effects:
- Graneli[9] showed that Prymnesium spp. will produce phycotoxins which kill competitors under nitrogen or phosphorus limitation.
- Fistarol et al.[10] found that Alexandrium spp. produce toxins which decrease the growth rate of other phytoplankton and change community composition.
- Prince et al.[11] showed that chemical exudates from the dinoflagellate Karenia brevis decreased the growth rate and sometimes killed competitor species by decreasing their photosynthetic efficiency and increasing membrane permeability.
List of known phycotoxins and mechanisms of action
Most characterized phycotoxins have some economic or health impact on humans. Other well-studied phycotoxins are potential or existing pharmaceuticals or have some use in cellular research. Therefore, our level of knowledge on individual toxins does not necessarily reflect their ecological relevance. Additionally, the mode of action and level of toxicity are effects that have been documented in macroorganisms (typically mice). These modes of action may be different in the pelagic marine environment. However, it is unlikely that the synthesis of complex and energetically expensive chemicals should be conserved over evolutionary time if they do not confer some advantage on the producer. Even if we do not yet know the effect of many toxins in their natural environment, their mere presence and impressive diversity indicates that they do serve some ecological purpose.
The phytoplankton species listed below do not encompass the entire range of known toxigenic species. There exists experimental evidence for phytoplankton species that have inhibitory effects on grazers or other phytoplankton species, but their toxins have not been identified.
Table generated using information from Cembella,[3] Shimizu[12]
| Toxin group | Toxin producing species | Class | Characteristics | Mode of action | Structure |
|---|---|---|---|---|---|
| Domoic acid | Pseudo-nitzschia spp. | Bacillariophyceae | Hydrophilic N-toxin | Glutamate receptor agonist | 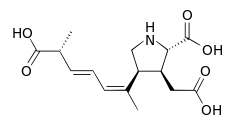 |
| Saxitoxins (neosaxitoxins, gonyautoxins) | Alexandrium spp., Pyrodinium bahamense, Gymnodinium catenatum | Dinophyceae | Hydrophilic N-toxin | Na+-channel blocker (site 1) | 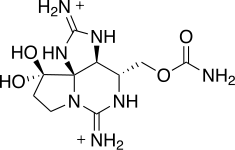 |
| Anabaena spp., Aphanizomenon spp., Cylindrospermopsis spp., Lyngbya spp., Planktothrix spp., Oscillatoria spp. | Cyanobacteria | ||||
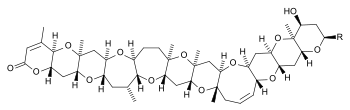
| Ciguatoxin | Gambierdiscus toxicus | Dinophyceae | Ladder-frame polyether | Na+-channel activator (site 5) | 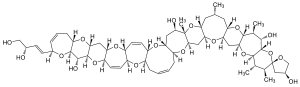 |
| Gambieric acid | Gambierdiscus toxicus | Dinophyceae | Ladder-frame polyether | ||
| Maitotoxins | Gambierdiscus toxicus | Dinophyceae | Ladder-frame polyether | Ca2+-channel effector | 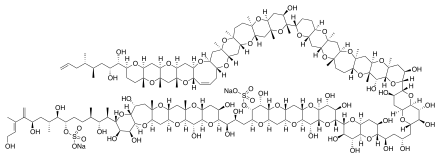 |
| Osterotoxin | Ostreopsis lenticularis | Dinophyceae | Ladder-frame polyether | Unknown | |
| Cooliatoxin | Coolia monotis | Dinophyceae | Ladder-frame polyether | Unknown | |
| Brevetoxins | Karenia brevis, K. brevi-sulcata | Dinophyceae | Ladder-frame polyether | Na+-channel activator (site 5) | 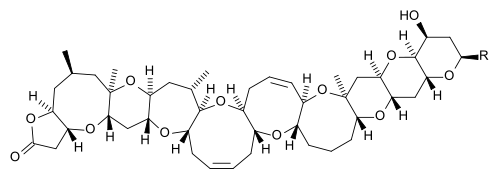
|
| Chatonella marina, C. antiqua, C. cf. verruculosa | Raphidophyceae | ||||
| Yessotoxins | Protoceratium reticulatum, Lingulodinium polyedrum | Dinophyceae | Ladder-frame polyether | Affects cyclic AMP, cytotoxic | 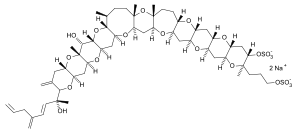 |
| Okadaic acid and dinophysistoxins | Dinophysis spp., Prorocentrum spp. | Dinophyceae | Linear polyether | Protein phosphatase inhibitor | 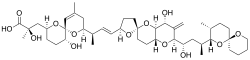 |
| Pectenotoxin | Dinophysis fortii, D. acuta | Dinophyceae | Macrocyclic polyether | Unknown, hepatotoxic | |
| Azaspriracids | Protoperidinium crassipes | Dinophyceae | Linear polyether | Unknown, neurotoxic | |
| Gymnodimine | Karenia selliformis | Dinophyceae | Macrolide | Unknown, potentially neurotoxic | |
| Prymnesins | Prymnesium parvum | Prymnesiophyceae | Linear polyether | Unknown, potential Ca2+-channel effector | |
| Spirolide | Alexandrium ostenfeldii | Dinophyceae | Macrocyclic polyether | Muscarinic receptor or cholinesterase inhibitor | |
| Ostreocin (palytoxin) | Ostreopsis siamensis | Dinophyceae | Linear polyether | Na+/K+ ATPase disruptor | 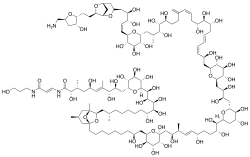 |
| Amphidinolide, Caribenolide | Amphidinium spp. | Dinophyceae | Macrocyclic polyether | Cytotoxic | |
| Goniodomin | Alexandrium spp. | Dinophyceae | Macrocyclic polyether | ||
| Prorocentrolide | Prorocentrium lima | Dinophyceae | Macrocyclic polyether | ||
| Scytophycins | Scytonema spp. | Cyanobacteria | Linear polyether | Cytotoxic | |
| Tolytoxin | Tolypothrix conglutinata var. colorata | Cyanobacteria | Linear polyether | Microfilament-depolymerizing agent | |
| Debromoaplysiatoxin | Lyngbya majuscula | Cyanobacteria | Linear polyether | Protein kinase C activator | 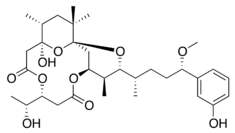 |
| Amphidinols, Amphiketide | Amphidinium spp. | Dinophyceae | Open-chain polyketides | Antifungal | |
| Majusculamides, Curacins | Lyngbya majuscula | Cyanobacteria | Open-chain polyketide | Microtubulin assembly inhibitor | |
| Bacillariolides | Pseudo-nitzschia multiseries | Bacillariophyceae | Eicosanoid | Phospholipase A2 inhibitor | |
| Lyngbyatoxins | Lyngbya majuscula | Cyanobacteria | Prenylated amino acid derivative | Protein kinase C activator | 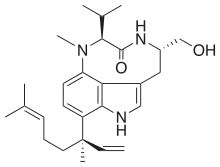 |
| Polyunsaturated aldehydes | Bacillariophyceae | Polyunsaturated aldehydes | Anti-mitotic, apoptosis | ||
| Euglenophycin | Euglena sanguinea | Euglenoidea | Polyketide | ||
| Karlotoxin[13] | Karlodinium veneficum | Dinophyceae | Polyketide / Polyether | 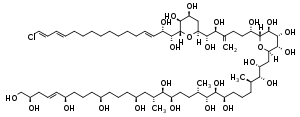 | |
| Karmitoxin[14] | Karlodinium armiger | Dinophyceae | Polyketide / Polyether | 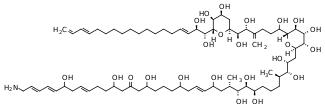 | |
Types of toxins
Excreted toxins
Excreted toxins may help deter predators and bacteria which are drawn in by phytoplankton waste products. Phytoplankton are known to excrete waste metabolites into the surrounding environment. This is a potential source of reduced nutrients and carbon for bacteria and may act as a signal for predators which can detect and follow kairomone gradients in their environment. Excreted toxins would seem most advantageous to the individual cell in their ability to keep predators and/or parasitic and algicidal bacteria at a distance. However, continuous toxin production and excretion carries a metabolic cost. For excreted toxins to be effective, they must have a low molecular weight to rapidly diffuse in the marine environment and to be energetically cheap to produce. However, excreted toxins may not actually repel larger motile predators because molecular diffusivity is slow and turbulence at the millimeter scale is large in water.[15] Excreted phycotoxins may act as repellents if their signal registers at the same speed as other signals that potential grazers can detect (kairomones), assuming both are encountered by a predator at the same time. Additionally, excreted toxins may be effective method of keeping harmful bacteria and other phytoplankton competitors outside of the phycotoxin producer's microzone of nutrients.
Contact toxins
Contact toxins are effective if they impact the grazer or harmful bacterium immediately after contact with the phytoplankton producer. These toxins are located at the cell surface and are typically classified as glycoproteins, glycolipids, or polypeptides. These toxins would have to be highly specific to their target receptors to be effective.
Post-ingestion toxins
In order for these types of toxins to take effect, post-ingestion toxin producers have to be consumed by a grazer. Post-ingestion toxins, also known as suicide toxins, are not beneficial to individual cells because unlike terrestrial plants, phytoplankton do not have sacrificial tissue. However, if internal toxins do result in the death, decrease growth rate, infertility, or deterrence of a predator the remaining representatives of the plankton community may benefit.[15] Community defense is most beneficial in a clonal population where toxigenic species are abundant, for example during a monospecific phytoplankton bloom.[16]
Chemical defense signal mechanisms
Table modified from Wolfe (2000)[15]
| Excreted toxins | Contact toxins | Post-ingestion toxins | |
|---|---|---|---|
| Molecules | Small molecules but varied structures; organic and amino acids, sugars, short-chain lipids and derivatives | Glycoproteins, glycolipids, polypeptides | Varied: toxicants or toxins |
| Toxin properties | Aqueous solubility, diffusivity, lability, toxicity | Specificity, toxicity | Toxicity or concentration |
| Toxin location | Aqueous environment | Cell surface | Cell interior |
| Effect or mode of action | Negative kinesis/taxis: repellent | Release following capture: deterrent | Subsequent inhibition of feeding or toxicity reduced digestibility or growth efficiency |
| Benefit level | Individual or population, including competitors | Individual | Genetically similar population |
Detection methods
It is technically difficult to identify and characterize a metabolite that is produced in low concentrations and is secreted into a fluid that contains a diversity of other metabolites. Allelopathy is very difficult to observe in the field (with the exception of harmful algal blooms) because phycotoxin production may be induced by a variety of environmental factors and may create a cascade of biotic and physical events, which are difficult to separate from direct allelopathic effects of one species on another. There are six points (similar in logic to Koch's postulates) that must be established to rigorously prove that one species is chemically inhibiting another in an ecological system[17]
- a pattern of inhibition of one species [...] by another must be shown
- the putative aggressor [species] must produce a toxin
- there must be a mode of toxin release from the [species] into the environment
- there must be a mode of toxin transport and/or accumulation in the environment
- the afflicted [species] must have some means of toxin uptake
- the observed pattern of inhibition cannot be explained solely by physical factors or other biotic factors, especially competition and herbivory:[3]
- concentrations which impact the target species must be environmentally realistic given rates of transport and diffusion in the aquatic environment
Few (if any) studies on phytoplankton toxins have attempted to rigorously meet all of these criteria. All methods of detecting phycotoxins involve extraction of the candidate toxin from a phytoplankton culture; therefore, it's important to determine whether the toxin is secreted into the media or stored in the phytoplankton cell. It's also important to know whether the target organism must be present to induce toxin synthesis.
Most commonly, the presence of a phycotoxin is verified by bioassay-guided fractionation.[16] The sample must be fractionated, or separated from the other metabolites and chemicals in the media using chromatography. These different fractions may be then tested on the target species to determine which sample causes the expected allelopathic symptom(s). This approach is useful for rapidly isolating an allelochemical whose structure is not known. However, bioassays have the potential to generate false positives. This may occur if the bioassay is not controlled properly. For example, in a mixed batch culture the target species may die or have reduced growth rates due to competition for nutrients, dissolved inorganic carbon, or pH levels which are too low for the target species.
Developments in genomics, transcriptomics, proteomics, and metabolomics are now yielding large volumes of biochemical data. "Metabolic profiling" allows for comparison between biologically active and inactive samples and identification of compounds present at low concentrations using mass spectrometry. These samples may then be compared by principal component analysis. Characterization of the compounds present in the active sample (but not in the inactive sample) may then be identified and characterized using standard methods in mass spectroscopy. Isotope labeling may also be used to identify the pathways used in phycotoxin biosynthesis.
See also
References
- Smetacek, V (2001). "A watery arms race". Nature. 411 (6839): 745. Bibcode:2001Natur.411..745S. doi:10.1038/35081210. PMID 11459035.
- Verity, PG; V Smetacek (1996). "Organism life cycles, predation, and the structure of marine pelaic ecosystems". Marine Ecology Progress Series. 130: 277–293. Bibcode:1996MEPS..130..277V. doi:10.3354/meps130277.
- Cembella, AD (2003). "Chemical ecology of eukaryotic microalgae in marine ecosystems". Phycologia. 42 (4): 420–447. doi:10.2216/i0031-8884-42-4-420.1. S2CID 83500149.
- Teegarden, GJ (1999). "Copepod grazing selection and particle discrimination on the basis of PSP toxin content". Marine Ecology Progress Series. 181: 163–176. Bibcode:1999MEPS..181..163T. doi:10.3354/meps181163.
- Miralto, A; et al. (1999). "The insidious effect of diatoms on copepod reproduction". Nature. 402 (6758): 173–176. Bibcode:1999Natur.402..173M. doi:10.1038/46023. S2CID 4318896.
- Bates, SS; DJ Douglas; GJ Doucette; C Leger (1995). "Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo-nitzschia multiseries". Natural Toxins. 3 (6): 428–435. doi:10.1002/nt.2620030605. PMID 8612005.
- Sieburth, JM (1960). "Acrylic acid, an "antibiotic" principle in Phaeocystis blooms in Antarctic waters". Science. 132 (3428): 676–677. Bibcode:1960Sci...132..676M. doi:10.1126/science.132.3428.676. PMID 14446452. S2CID 41386593.
- Slezak, DM; S Puskaric; GJ Herndl (1994). "Potential role of acrylic acid in bacterioplankton communities in the sea" (PDF). Marine Ecology Progress Series. 105: 191–197. Bibcode:1994MEPS..105..191S. doi:10.3354/meps105191.
- Graneli, E (2006). "Kill your enemies and eat them with the help of your toxins: an algal strategy". African Journal of Marine Science. 28 (2): 331–336. Bibcode:2006AfJMS..28..331G. doi:10.2989/18142320609504172. S2CID 84323469.
- Fistarol, GA; C Legrand; E Selander; C Hummert; W Stolte; E Graneli (2004). "Allelopathy in Alexandrium spp.: effect on a natural plankton community and on algal monocultures". Aquatic Microbial Ecology. 35: 45–56. doi:10.3354/ame035045.
- Prince, EK; TL Myers; J Kubanek (2008). "Effects of harmful algal blooms on competitors: Allelopathic mechanisms of the red tide dinoflagellate Karenia brevis". Limnology and Oceanography. 53 (2): 531–541. Bibcode:2008LimOc..53..531P. doi:10.4319/lo.2008.53.2.0531.
- Shimizu, Y (1996). "Microbial metabolites: a new perspective". Annual Review of Microbiology. 50: 431–465. doi:10.1146/annurev.micro.50.1.431. PMID 8905087.
- Peng, Jiangnan; Place, Allen R.; Yoshida, Wesley; Anklin, Clemens; Hamann, Mark T. (2010-03-17). "Structure and Absolute Configuration of Karlotoxin-2, an Ichthyotoxin from the Marine DinoflagellateKarlodinium veneficum". Journal of the American Chemical Society. American Chemical Society (ACS). 132 (10): 3277–3279. doi:10.1021/ja9091853. ISSN 0002-7863. PMC 2836391. PMID 20155901.
- Rasmussen, Silas Anselm; Binzer, Sofie Bjørnholt; Hoeck, Casper; Meier, Sebastian; de Medeiros, Livia Soman; Andersen, Nikolaj Gedsted; Place, Allen; Nielsen, Kristian Fog; Hansen, Per Juel; Larsen, Thomas Ostenfeld (2017-04-05). "Karmitoxin: An Amine-Containing Polyhydroxy-Polyene Toxin from the Marine Dinoflagellate Karlodinium armiger". Journal of Natural Products. American Chemical Society (ACS). 80 (5): 1287–1293. doi:10.1021/acs.jnatprod.6b00860. ISSN 0163-3864. PMC 6446557. PMID 28379705.
- Wolfe, GV (2000). "The chemical defense ecology of marine unicellular plankton: constraints, mechanisms, and impacts". The Biological Bulletin. 198 (2): 225–244. CiteSeerX 10.1.1.317.7878. doi:10.2307/1542526. JSTOR 1542526. PMID 10786943.
- Pohnert, G; M Steinke; R Tollrian (2007). "Chemical cues, defense metabolites and the shaping of pelagic interspecific interactions". Trends in Ecology & Evolution. 22 (4): 198–204. doi:10.1016/j.tree.2007.01.005. PMID 17275948.
- Willis, RJ (1985). "The historical bases of the concept of allelopathy". Journal of the History of Biology. 18 (1): 71–102. doi:10.1007/BF00127958. S2CID 83639846.
Further reading
- Ianora, A, et al. "The H.T. Odum Synethesis Essay New trends in marine chemical ecology." Estuaries and coasts 29 (2006): 531–551.
- Ianora, A, et al. "The relevance of marine chemical ecology to plankton and ecosystem function: An emerging field." Marine Drugs 9 (2011): 1625–1648.
- Taylor, P, and JH Landsberg. "The effects of harmful algal blooms on aquatic organisms." Reviews in Fisheries Science 10 (2010): 113–390.
- Weissburg, MJ. "The fluid dynamical context of chemosensory behavior." The Biological Bulletin 198 (2000): 188–202.
- Legrand, C, K Rengefors, GO Fistarol, and E Graneli. "Allelopathy in phytoplankton - biochemical, ecological and evolutionary aspects." Phycologia 42 (2003): 406–419.