Scanning vibrating electrode technique
Scanning vibrating electrode technique (SVET), also known as vibrating probe within the field of biology, is a scanning probe microscopy (SPM) technique which visualizes electrochemical processes at a sample. It was originally introduced in 1974 by Jaffe and Nuccitelli to investigate the electrical current densities near living cells.[1] Starting in the 1980s Hugh Isaacs began to apply SVET to a number of different corrosion studies.[2] SVET measures local current density distributions in the solution above the sample of interest, to map electrochemical processes in situ as they occur. It utilizes a probe, vibrating perpendicular to the sample of interest, to enhance the measured signal.[1] It is related to scanning ion-selective electrode technique (SIET), which can be used with SVET in corrosion studies,[3] and scanning reference electrode technique (SRET), which is a precursor to SVET.[4]
History
Scanning vibrating electrode technique was originally introduced to sensitively measure extracellular currents by Jaffe and Nuccitelli in 1974.[1] Jaffe and Nuccitelli then demonstrated the ability of the technique through the measurement of the extracellular currents involved with amputated and re-generating newt limbs,[5] developmental currents of chick embryos,[6] and the electrical currents associated with amoeboid movement.[7]
In corrosion, the scanning reference electrode technique (SRET) existed as the precursor to SVET, and was first introduced commercially and trademarked by Uniscan Instruments,[8] now part of Bio-Logic Science Instruments.[9] SRET is an in situ technique in which a reference electrode is scanned near a sample surface to map the potential distribution in the electrolyte above the sample. Using SRET it is possible to determine the anodic and cathodic sites of a corroding sample without the probe altering the corrosion process.[10] SVET was first applied to and developed for the local investigation of corrosion processes by Hugh Isaacs.[2]
Principle of Operation
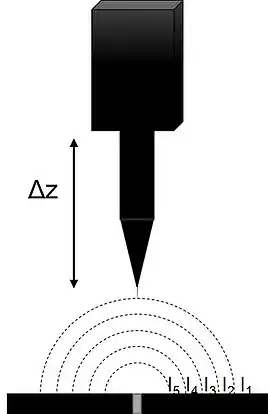
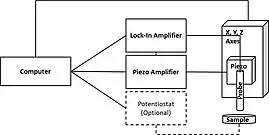
SVET measures the currents associated with a sample in solution with natural electrochemical activity, or which is biased to force electrochemical activity. In both cases the current radiates into solution from the active regions of the sample. In a typical SVET instrument the probe is mounted on a piezoelectric vibrator on and x,y stage. The probe is vibrated perpendicular to the plane of the sample resulting in the measurement of an ac signal. The resulting ac signal is detected and demodulated using an input phase angle by a lock-in amplifier to produce a dc signal.[1][11][12] The input phase angle is typically found by manually adjusting the phase input of the Lock-in Amplifier until there is no response, 90 degrees is then added to determine the optimum phase.[13] The reference phase can also be found automatically by some commercial instruments.[14] The demodulated dc signal which results can then be plotted to reflect the local activity distribution.
In SVET, the probe vibration results in a more sensitive measurement than its non-vibrating predecessors,[1] as well as giving rise to an improvement of the signal-to-noise ratio.[13] The probe vibration does not affect the process under study under normal experimental conditions.[15][16]
The SVET signal is affected by a number of factors including the probe to sample distance, solution conductivity, and the SVET probe. The signal strength in a SVET measurement is influenced by the probe to sample distance. When all other variables are equal a smaller probe to sample distance will result in the measurement of a higher magnitude signal.[17] The solution conductivity affects the signal strength in SVET measurements. With increasing solution conductivity, the signal strength of the SVET measurement decreases.[18]
Applications
Corrosion is a major application area in for SVET. SVET is used to follow the corrosion process and provide information not possible from any other technique.[19] In corrosion it has been used to investigate a variety of processes including, but not limited to, local corrosion, self-healing coatings, Self-Assembled Monolayers (SAMs). SVET has also been used to investigate the effect of different local features on the corrosion properties of a system. For example, using SVET, the influence of the grains and grain boundaries of X70 was measured. A difference in current densities existed between the grains and grain boundaries with the SVET data suggesting the grain was anodic, and the boundary relatively cathodic.[20] Through the use of SVET it has been possible to investigate the effect of changing the aluminum spacer width on the galvanic coupling between steel and magnesium, a pairing which can be found on automobiles. Increasing the spacer width reduced the coupling between magnesium and steel.[21] More generally localized corrosion processes have been followed using SVET. For a variety of systems it has been possible to use SVET to follow the corrosion front as it moves across the sample over extended periods, providing insight into the corrosion mechanism.[22][23][24] A number of groups have used SVET to analyze the efficiency of self-healing coatings, mapping the changes in surface activity over time. When SVET measurements of the bare metals are compared to the same metal with the smart coating it can be seen that the current density is lower for the coated surface. Furthermore, when a defect is made in the smart coating the current over the defect can be seen to decrease as the coating recovers.[25][26][27] Mekhalif et. al. have performed a number of studies on SAMs formed on different metals to investigate their corrosion inhibition using SVET. The SVET studies revealed that the bare surfaces experience corrosion, with inhomogeneous activity measured by SVET. SVET was then used to investigate the effect of modification time,[28] and exposure to corrosive solution.[29] When a defect free SAM was investigated SVET showed homogeneous activity.[30][31]
In the field of biology the vibrating probe technique has been used to investigate a variety of processes. Vibrating probe measurements of lung cancer tumor cells have shown that the electric fields above the tumor cell were statistically larger than those measured over the intact epithelium, with the tumor cell behaving as the anode. Furthermore, it was noted that the application of an electric field resulted in the migration of the tumor cells.[32] Using vibrating probe, the electrical currents involved in the biological processes occurring at leaves have been measured. Through vibrating probe it has been possible to correlate electrical currents with the stomatal aperture, suggesting that stomatal opening was related to proton efflux.[33] Based on this work further vibrating probe measurements also indicated a relationship between the photosynthetic activity of a plant and the flow of electrical current on its leaf surfaces, with the measured current changing when it was exposed to different types of light and dark.[34][35] As a final example, the vibrating probe technique has been used in the investigation of currents associated with wounding in plants and animals. A vibrating probe measurement of maize roots found that large inward currents were associated with wounding of the root, with the current decreasing in magnitude away from the center of the wound.[36] When similar experiments were performed on rat skin wounds, large outward currents were measured at the wound, with the strongest current measured at the wound edge.[37] The ability of the vibrating probe to investigate wounding has even lead to the development of a hand held prototype vibrating probe device for use.[38]
SVET has been used to investigate the photoconductive nature of semiconductor materials, by following changes in current density related to photoelectrochemical reactions.[39] Using SVET the lithium/organic electrolyte interface, as in lithium battery systems has also been investigated.[40]
Although SVET has almost exclusively been applied for the measurement of samples in aqueous environments, its application in non-aqueous environments has recently been demonstrated by Bastos et al.[41]
References
- Jaffe, L. F. (1974-11-01). "An Ultrasensitive Vibrating Probe for Measuring Steady Extracellular Currents". The Journal of Cell Biology. 63 (2): 614–628. doi:10.1083/jcb.63.2.614. ISSN 0021-9525. PMC 2110946. PMID 4421919.
- Isaacs, H. S. (1988). "Initiation of Stress Corrosion Cracking of Sensitized Type 304 Stainless Steel in Dilute Thiosulfate Solution". Journal of the Electrochemical Society. 135 (9): 2180–2183. Bibcode:1988JElS..135.2180I. doi:10.1149/1.2096235.
- Upadhyay, Vinod; Battocchi, Dante (October 2016). "Localized electrochemical characterization of organic coatings: A brief review". Progress in Organic Coatings. 99: 365–377. doi:10.1016/j.porgcoat.2016.06.012. ISSN 0300-9440.
- Ramos, Rogelio; Zlatev, Roumen; Stoytcheva, Margarita; Valdez, Benjamin; Kiyota, Sayuri (2010). "Novel SVET Approach and its Application for Rapid Pitting Corrosion Studies of Chromatized Aerospatiale Aluminum Alloy". ECS Transactions. 29 (1). ECS: 23–31. Bibcode:2010ECSTr..29a..23R. doi:10.1149/1.3532300. S2CID 139071821.
{{cite journal}}: Cite journal requires|journal=(help) - Borgens, R. B.; Vanable, J. W.; Jaffe, L. F. (1977-10-01). "Bioelectricity and regeneration: large currents leave the stumps of regenerating newt limbs". Proceedings of the National Academy of Sciences. 74 (10): 4528–4532. Bibcode:1977PNAS...74.4528B. doi:10.1073/pnas.74.10.4528. ISSN 0027-8424. PMC 431978. PMID 270701.
- Jaffe, L.; Stern, C. (1979-11-02). "Strong electrical currents leave the primitive streak of chick embryos". Science. 206 (4418): 569–571. Bibcode:1979Sci...206..569J. doi:10.1126/science.573921. ISSN 0036-8075. PMID 573921.
- Nuccitelli, R. (1977-06-01). "Relations between ameboid movement and membrane-controlled electrical currents". The Journal of General Physiology. 69 (6): 743–763. doi:10.1085/jgp.69.6.743. ISSN 0022-1295. PMC 2215338. PMID 19555.
- Borgwarth, K.; Ebling, D.; Heinze, J. (1995). "Applications of scanning ultra micro electrodes for studies on surface conductivity". Electrochimica Acta. 40 (10): 1455–1460. doi:10.1016/0013-4686(95)99707-3. ISSN 0013-4686.
- "Bio-Logic Science Instruments". Bio-Logic Science Instruments. Retrieved 2019-05-13.
- Isaacs, HS; Vyas, B (1981), "Scanning Reference Electrode Techniques in Localized Corrosion", Electrochemical Corrosion Testing, ASTM International, pp. 3–3–31, doi:10.1520/stp28024s, ISBN 9780803107045
- Nawata, Tomoki (1984). "A Simple Method for Making a Vibrating Probe System". Plant and Cell Physiology. doi:10.1093/oxfordjournals.pcp.a076795. ISSN 1471-9053.
- Dorn, A.; Weisenseel, M. H. (1982). "Advances in vibrating probe techniques". Protoplasma. 113 (2): 89–96. doi:10.1007/bf01281996. ISSN 0033-183X. S2CID 9840545.
- Reid, Brian; Nuccitelli, Richard; Zhao, Min (2007). "Non-invasive measurement of bioelectric currents with a vibrating probe". Nature Protocols. 2 (3): 661–669. doi:10.1038/nprot.2007.91. ISSN 1754-2189. PMID 17406628. S2CID 15237787.
- "M470 - SVP / SVET". Bio-Logic Science Instruments. Retrieved 2019-03-27.
- Ferrier, J.; Lucas, W.J. (1986). "Ion transport and the vibrating probe". Biophysical Journal. 49 (4): 803–807. Bibcode:1986BpJ....49..803F. doi:10.1016/s0006-3495(86)83708-0. ISSN 0006-3495. PMC 1329531. PMID 2424512.
- Bastos, A.C.; Quevedo, M.C.; Ferreira, M.G.S. (2015). "The influence of vibration and probe movement on SVET measurements". Corrosion Science. 92: 309–314. doi:10.1016/j.corsci.2014.10.038. ISSN 0010-938X.
- Akid, R; Garma, M (2004). "Scanning vibrating reference electrode technique: a calibration study to evaluate the optimum operating parameters for maximum signal detection of point source activity". Electrochimica Acta. 49 (17–18): 2871–2879. doi:10.1016/j.electacta.2004.01.069. ISSN 0013-4686.
- Dzib‐Pérez, L.; González‐Sánchez, J.; Malo, J.M.; Rodríguez, F.J. (2009-01-09). "The effect of test conditions on the sensitivity and resolution of SRET signal response". Anti-Corrosion Methods and Materials. 56 (1): 18–27. doi:10.1108/00035590910923428. ISSN 0003-5599.
- Bastos, A. C.; Quevedo, M. C.; Karavai, O. V.; Ferreira, M. G. S. (2017). "Review—On the Application of the Scanning Vibrating Electrode Technique (SVET) to Corrosion Research". Journal of the Electrochemical Society. 164 (14): C973–C990. doi:10.1149/2.0431714jes. ISSN 0013-4651. S2CID 103833880.
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. (2010). "In-situ characterization of the electrochemistry of grain and grain boundary of an X70 steel in a near-neutral pH solution". Electrochemistry Communications. 12 (7): 936–938. doi:10.1016/j.elecom.2010.04.025. ISSN 1388-2481.
- Deshpande, Kiran B. (2012). "Effect of aluminium spacer on galvanic corrosion between magnesium and mild steel using numerical model and SVET experiments". Corrosion Science. 62: 184–191. doi:10.1016/j.corsci.2012.05.013. ISSN 0010-938X.
- Cain, T.W.; Glover, C.F.; Scully, J.R. (2019). "The corrosion of solid solution Mg-Sn binary alloys in NaCl solutions". Electrochimica Acta. 297: 564–575. doi:10.1016/j.electacta.2018.11.118. S2CID 105480590.
- Andreatta, Francesco; Rodriguez, Justine; Mouanga, Maixent; Lanzutti, Alex; Fedrizzi, Lorenzo; Olivier, Marjorie G. (2018-12-27). "Corrosion protection by zinc-magnesium coatings on steel studied by electrochemical methods". Materials and Corrosion. 70 (5): 793–801. doi:10.1002/maco.201810554. S2CID 104399009.
- Laferrere, Alice; Burrows, Robert; Glover, Carol; Clark, Ronald Nuuchin; Payton, Oliver; Picco, Loren; Moore, Stacy; Williams, Geraint (2017-10-09). "In situ imaging of corrosion processes in nuclear fuel cladding" (PDF). Corrosion Engineering, Science and Technology. 52 (8): 596–604. doi:10.1080/1478422x.2017.1344038. ISSN 1478-422X. S2CID 55472047.
- Stankiewicz, Alicja; Kefallinou, Zoi; Mordarski, Grzegorz; Jagoda, Zofia; Spencer, Ben (2019). "Surface functionalisation by the introduction of self-healing properties into electroless Ni-P coatings". Electrochimica Acta. 297: 427–434. doi:10.1016/j.electacta.2018.12.026. ISSN 0013-4686. S2CID 104433006.
- Wang, MingDong; Liu, MengYang; Fu, JiaJun (2015). "An intelligent anticorrosion coating based on pH-responsive smart nanocontainers fabricated via a facile method for protection of carbon steel". Journal of Materials Chemistry A. 3 (12): 6423–6431. doi:10.1039/c5ta00417a. ISSN 2050-7488.
- Adsul, Swapnil H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, Shirish H.; Subasri, R. (2017). "Self-healing ability of nanoclay-based hybrid sol-gel coatings on magnesium alloy AZ91D". Surface and Coatings Technology. 309: 609–620. doi:10.1016/j.surfcoat.2016.12.018. ISSN 0257-8972.
- Berger, François; Delhalle, Joseph; Mekhalif, Zineb (2009). "Undec-10-ene-1-thiol multifunctional molecular layer as a junction between metallic zinc and polymer coatings on steel". Electrochimica Acta. 54 (26): 6464–6471. doi:10.1016/j.electacta.2009.06.021. ISSN 0013-4686.
- Berger, François; Delhalle, Joseph; Mekhalif, Zineb (2008). "Hybrid coating on steel: ZnNi electrodeposition and surface modification with organothiols and diazonium salts". Electrochimica Acta. 53 (6): 2852–2861. doi:10.1016/j.electacta.2007.10.067. ISSN 0013-4686.
- Berger, François; Delhalle, Joseph; Mekhalif, Zineb (2010). "Self-assembled bilayers based on organothiol and organotrimethoxysilane on zinc platform". Applied Surface Science. 256 (23): 7131–7137. Bibcode:2010ApSS..256.7131B. doi:10.1016/j.apsusc.2010.05.039. ISSN 0169-4332.
- Laffineur, F.; Auguste, D.; Plumier, F.; Pirlot, C.; Hevesi, L.; Delhalle, J.; Mekhalif, Z. (2004). "Comparison between CH3(CH2)15SH and CF3(CF2)3(CH2)11SH Monolayers on Electrodeposited Silver". Langmuir. 20 (8): 3240–3245. doi:10.1021/la035851+. ISSN 0743-7463. PMID 15875853.
- Li, Li; Zhang, Kejun; Lu, Conghua; Sun, Qin; Zhao, Sanjun; Jiao, Lin; Han, Rui; Lin, Caiyu; Jiang, Jianxin (2018-06-15). "Correction: Caveolin-1-mediated STAT3 activation determines electrotaxis of human lung cancer cells". Oncotarget. 9 (46): 28291. doi:10.18632/oncotarget.25675. ISSN 1949-2553. PMC 6021323. PMID 29963279.
- Penny, M. G.; Kelday, L. S.; Bowling, D. J. F. (1976). "Active chloride transport in the leaf epidermis of Commelina communis in relation to stomatal activity". Planta. 130 (3): 291–294. doi:10.1007/bf00387835. ISSN 0032-0935. PMID 24424642. S2CID 3216411.
- Weisenseel, M. H.; Linder, B. (1990). "Polar current patterns in the leaves of the aquatic angiospermElodea Canadensis". Protoplasma. 157 (1–3): 193–202. doi:10.1007/BF01322652. ISSN 0033-183X. S2CID 38050334.
- Lee, Joon Sang (2006). "Response to red and blue lights by electrical currents on the surface of intact leaves". Journal of Plant Biology. 49 (2): 186–192. doi:10.1007/bf03031016. ISSN 1226-9239. S2CID 25520758.
- Meyer, A. J.; Weisenseel, M. H. (1997-07-01). "Wound-Induced Changes of Membrane Voltage, Endogenous Currents, and Ion Fluxes in Primary Roots of Maize". Plant Physiology. 114 (3): 989–998. doi:10.1104/pp.114.3.989. ISSN 0032-0889. PMC 158387. PMID 12223755.
- Li, Li; Gu, Wei; Du, Juan; Reid, Brian; Deng, Xianjian; Liu, Zhidai; Zong, Zhaowen; Wang, Haiyan; Yao, Bo (2012-10-19). "Electric fields guide migration of epidermal stem cells and promote skin wound healing". Wound Repair and Regeneration. 20 (6): 840–851. doi:10.1111/j.1524-475x.2012.00829.x. ISSN 1067-1927. PMID 23082865. S2CID 23921011.
- Barker, A. T. (1981). "Measurement of direct currents in biological fluids". Medical & Biological Engineering & Computing. 19 (4): 507–508. doi:10.1007/bf02441322. ISSN 0140-0118. PMID 7321622. S2CID 19376455.
- Maltanava, Hanna M.; Poznyak, Sergey K.; Andreeva, Daria V.; Quevedo, Marcela C.; Bastos, Alexandre C.; Tedim, João; Ferreira, Mário G. S.; Skorb, Ekaterina V. (2017-07-07). "Light-Induced Proton Pumping with a Semiconductor: Vision for Photoproton Lateral Separation and Robust Manipulation" (PDF). ACS Applied Materials & Interfaces. 9 (28): 24282–24289. doi:10.1021/acsami.7b05209. hdl:10773/24930. ISSN 1944-8244. PMID 28654237.
- Ishikawa, Masashi (1994). "In Situ Scanning Vibrating Electrode Technique for the Characterization of Interface Between Lithium Electrode and Electrolytes Containing Additives". Journal of the Electrochemical Society. 141 (12): L159–L161. Bibcode:1994JElS..141L.159I. doi:10.1149/1.2059378. ISSN 0013-4651.
- Bastos, A.C.; Quevedo, M.C.; Ferreira, M.G.S. (2016). "Preliminary research on the use of SVET in non-aqueous media". Electrochimica Acta. 202: 310–315. doi:10.1016/j.electacta.2015.12.107. ISSN 0013-4686.