Mepivacaine
Mepivacaine /mɛˈpɪvəkeɪn/ is a local anesthetic[1] of the amide type. Mepivacaine has a reasonably rapid onset (less rapid than that of procaine) and medium duration of action (longer than that of procaine)[2][3] and is marketed under various trade names including Carbocaine and Polocaine.
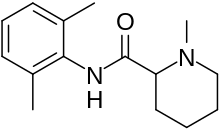 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a603026 |
| Pregnancy category |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.313 |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mepivacaine became available in the United States in the 1960s.
Mepivacaine is used in any infiltration and local anesthesia.
It is supplied as the hydrochloride salt of the racemate,[4] which consists of R(-)-mepivacaine and S(+)-mepivacaine in equal proportions. These two enantiomers have markedly different pharmacokinetic properties.[4]
Mepivacaine was originally synthesized in Sweden at the laboratory of Bofors Nobelkrut in 1956.[5]
References
- Porto GG, Vasconcelos BC, Gomes AC, Albert D (January 2007). "Evaluation of lidocaine and mepivacaine for inferior third molar surgery" (PDF). Medicina oral, patología oral y cirugía bucal. 12 (1): E60–4. PMID 17195831.
- "Procaine". go.drugbank.com. Retrieved 2023-06-27.
- "Mepivacaine". go.drugbank.com. Retrieved 2023-06-27.
- Burm AG, Cohen IM, van Kleef JW, Vletter AA, Olieman W, Groen K (January 1997). "Pharmacokinetics of the enantiomers of mepivacaine after intravenous administration of the racemate in volunteers". Anesthesia & Analgesia. 84 (1): 85–9. doi:10.1097/00000539-199701000-00016. PMID 8989005. S2CID 22363370.
- Castrén, J.A. (1963). "A clinical evaluation of mepivacaine (Carbocain) in ocular surgery". Acta Ophthalmologica. 41 (3): 262–9. doi:10.1111/j.1755-3768.1963.tb02436.x. PMID 14047466. S2CID 32119846.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.