Salicyl alcohol
Salicyl alcohol (saligenin) is an organic compound with the formula C6H4(OH)(CH2OH). It is a white solid that is used as a precursor in organic synthesis.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Hydroxymethyl)phenol | |
| Other names
2-Hydroxybenzyl alcohol, Salicain, Diathesin, Saligenin, Saligenol, Salicyl alcohol, α,2-Toluenediol, o-Methylolphenol, 2-Methylolphenol, Salicylic alcohol[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.782 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H8O2 | |
| Molar mass | 124.139 g·mol−1 |
| Density | 1.16 g/cm3 |
| Melting point | 86 °C (187 °F; 359 K) |
| Boiling point | 267 °C (513 °F; 540 K) |
| 67g/L at 22 °C[2] | |
| -76.9·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | 134 °C[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chemical synthesis
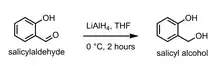
Reaction scheme for the reduction of salicylaldehyde to salicyl alcohol via lithium aluminium hydride.
Salicyl alcohol can be prepared through the reduction of salicylaldehyde via lithium aluminium hydride (LAH) in THF.
Applications
Chemical sweeteners were formed by acetal formation with e.g. isovanillin (Cmp4).[4]
Salicyl alcohol appears as a pharmacophore in several notable β2-adrenoceptor agonists (e.g. salbutamol), as well as in synthetic estrone analogs, e.g. CID:22940780 or CID:154236944.
Biosynthesis
Salicyl alcohol is the precursor of salicylic acid.[5] It is formed from salicin by enzymatic hydrolysis by Salicyl-alcohol beta-D-glucosyltransferase or by acid hydrolysis.
See also
- Gastrodigenin (4-hydroxybenzyl alcohol)
- Discovery and development of beta2 agonists
References
- "2-Hydroxybenzyl alcohol". chemicalbook.com.
- "salicylic alcohol". chemspider.com.
- Vishwakarma Singh, Mini Porinchu, Punitha Vedantham, Pramod K. Sahu1 (2005). "Synthesis of 9-Spiroepoxy-endo-Tricyclo[5.2.2.0]undeca-4,10-dien-8-one". Organic Syntheses. 81: 171. doi:10.15227/orgsyn.081.0171.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bassoli, Angela; Merlini, Lucio; Morini, Gabriella (2002). "Isovanillyl sweeteners. From molecules to receptors". Pure and Applied Chemistry. 74 (7): 1181–1187. doi:10.1351/pac200274071181. ISSN 1365-3075. S2CID 53554546.
- Seo, Eun-Seong; Lee, Jin-Ha; Park, Ji-Young; Kim, Doman; Han, Ho-Jae; Robyt, John F. (2005). "Enzymatic synthesis and anti-coagulant effect of salicin analogs by using the Leuconostoc mesenteroides glucansucrase acceptor reaction". Journal of Biotechnology. 117 (1): 31–38. doi:10.1016/j.jbiotec.2004.10.013. PMID 15831245.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.