Laurite
Laurite is an opaque black, metallic ruthenium sulfide mineral with formula: RuS2. It crystallizes in the isometric system. It is in the pyrite structural group. Though it's been found in many localities worldwide, it is extremely rare.
| Laurite | |
|---|---|
 | |
| General | |
| Category | Sulfide mineral |
| Formula (repeating unit) | RuS2 |
| Strunz classification | 2.EB.05a |
| Crystal system | Cubic |
| Crystal class | Diploidal (m3) H-M symbol: (P 2/m 3) |
| Space group | Pa3 |
| Unit cell | a = 5.61 Å; Z = 4 |
| Structure | |
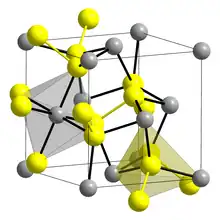
Crystal structure of Laurite S Ru | |
| Identification | |
| Color | Iron-black; white to gray or bluish in polished section |
| Crystal habit | As octahedral, cubic, and pyritohedral crystals or as rounded grains and inclusions |
| Cleavage | Perfect on {111} |
| Fracture | Subconchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 7.5 |
| Luster | Metallic |
| Streak | Dark gray |
| Diaphaneity | Opaque |
| Specific gravity | 6.43 |
| Density | 6.43 g/cm3 (Measured) 6.39 g/cm3 (Calculated) |
| Optical properties | Isotropic and opaque |
| References | [1][2][3] |
Laurite has a Mohs hardness of 7.5 and a specific gravity of 6.43. It can contain osmium, rhodium, iridium, and iron substituting for the ruthenium.[2] The sulfur is present as the disulfide ion, S2−2, so the ruthenium is in the Ru(II) oxidation state.[4]
Discovery and occurrence
It was discovered in 1866 in Borneo, Malaysia and named for Laurie, the wife of Charles A. Joy, an American chemist.[2] It occurs in ultramafic magmatic cumulate deposits and sedimentary placer deposits derived from them. It occurs associated with cooperite, braggite, sperrylite, other minerals of the platinum group elements and chromite.[1]
Synthetic RuS2 is a highly active catalyst for hydrodesulfurization.[5]
References
- Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (2005). "Laurite" (PDF). Handbook of Mineralogy. Mineral Data Publishing. Retrieved 14 March 2022.
- Laurite, Mindat.org
- "Laurite Mineral Data". Webmineral.com.
- Cocco, R.A.; Tatarchuk, B.J. (1989). "Effects of presulfidization on the selectivity and surface structure of ruthenium catalysts". Langmuir. 5 (6): 1309–1315. doi:10.1021/la00090a005. Retrieved 20 June 2022.
- Chianelli, R. R.; Berhault, G.; Raybaud, P.; Kasztelan, S.; Hafner, J.; Toulhoat, H. (2002). "Periodic Trends in Hydrodesulfurization: in Support of the Sabatier Principle". Appl. Catal., A. 227 (1–2): 83–96. doi:10.1016/S0926-860X(01)00924-3.