Protonated ozone
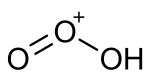
Protonated ozone is a hydrogen polyoxide having the molecular formula HO+3 (also written O3H+). It is a cationic structure consisting of an ozone unit with a hydrogen atom attached to one end. This substance is proposed to exist as an intermediate in several interstellar, atmospheric,and synthetic chemical processes.[1] It has been synthesized in mass spectrometer experiments by protonation of ozone using various strong acids.[2] Related experiments have used it as the precursor for generating hydrogen ozonide.[3]
 | |
| Names | |
|---|---|
| IUPAC name
Hydroxy(oxo)oxidanium | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| HO3+ | |
| Molar mass | 49.004 g·mol−1 |
| Conjugate base | Ozone |
| Related compounds | |
Related hydrogen polyoxides |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Ceotto, Michele; Gianturco, Franco A.; Hirst, David M. (1999). "Protonated Ozone: Structure, Energetics, and Nonadiabatic Effects". J. Phys. Chem. A. 103 (48): 9984–9994. Bibcode:1999JPCA..103.9984C. doi:10.1021/jp9923769.
- Cacace, Fulvio; Speranza, Maurizio (8 July 1994). "Protonated Ozone: Experimental Detection of O3H+ and Evaluation of the Proton Affinity of Ozone". Science. 265 (5169): 208–209. Bibcode:1994Sci...265..208C. doi:10.1126/science.265.5169.208. PMID 17750658. S2CID 29517377.
- Cacace, Fulvio; de Petris, Giulia; Pepi, F.; Troiani, Anna (2 July 1999). "Experimental Detection of Hydrogen Trioxide". Science. 285 (5424): 81–82. doi:10.1126/science.285.5424.81. PMID 10390365.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.