Potassium ethyl xanthate
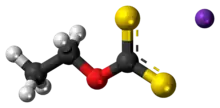
Potassium ethyl xanthate (KEX) is an organosulfur compound with the chemical formula CH3CH2OCS2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. It is a potassium salt of ethyl xanthic acid.
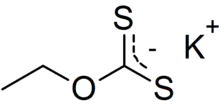 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Potassium O-ethylcarbonodithioate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.946 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3CH2OCS2K | |
| Molar mass | 160.29 g·mol−1 |
| Appearance | Pale yellow powder |
| Density | 1.263 g/cm3[1] |
| Melting point | 225 to 226 °C (437 to 439 °F; 498 to 499 K) |
| Boiling point | decomposes |
| Acidity (pKa) | approximately 1.6 |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H228, H302, H315, H319, H332, H335 | |
| P210, P240, P241, P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P370+P378, P403+P233, P405, P501 | |
| Related compounds | |
Other cations |
Sodium ethyl xanthate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production and properties
Xanthate salts are prepared by the action of alkoxides on carbon disulfide. The alkoxide is often generated in situ from potassium hydroxide:[2]
- CH3CH2OH + CS2 + KOH → CH3CH2OCS2K + H2O
Potassium ethyl xanthate is a pale yellow powder that is stable at high pH, but rapidly hydrolyses below pH = 9:
- CH3CH2OCS2K + H+ → CH3CH2OH + CS2 + K+
Oxidation gives diethyl dixanthogen disulfide:
- 4 CH3CH2OCS2K + 2 H2O + O2 → 2 (CH3CH2OCS2)2 + 4 KOH
KEX is a source of ethylxanthate coordination complexes. For example (CH3CH2OCS2)3M have been prepared from KEX for M = Cr, In, Co.[3]
Applications
Potassium ethyl xanthate is used in the mining industry as flotation agent for extraction of the ores of copper, nickel, and silver.[4] The method exploits the affinity of these "soft" metals for the organosulfur ligand.
Potassium xanthate is a useful reagent for preparing xanthate esters from alkyl and aryl halides. The resulting xanthate esters are useful intermediates in organic synthesis.[5]
References
- Report 5 (1995) p. 5
- This report gives a detailed recipe for potassium ethyl xanthate: Charles C. Price, Gardner W. Stacy (1948). "p-Nitrophenyl) Sulfide". Organic Syntheses. 28: 82. doi:10.15227/orgsyn.028.0082.
- Galsbøl, F.; Schäffer, C. E. (1967). "Tris (O-Ethyldithiocarbonato) Complexes of Tripositive Chromium, Indium, and Cobalt". Inorganic Syntheses. 10: 42–49. doi:10.1002/9780470132418.ch6.
- Kathrin-Maria Roy (2005). "Xanthates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_423. ISBN 3527306730.
- One of several procedures using xanthate esters: Fabien Gagosz and Samir Z. Zard (1948). "A Xanthate-Transfer Approach to α-Trifluoromethylamines". Organic Syntheses. 84: 32.; Collective Volume, vol. 11, p. 212