Piceol
Piceol is a phenolic compound found in the needles and in mycorrhizal roots of Norway spruces (Picea abies).[1][2] Picein is the glucoside of piceol.[3]
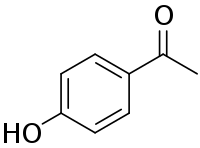 Chemical structure of piceol | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(4-Hydroxyphenyl)ethan-1-one | |
| Other names
1-(4-Hydroxyphenyl)ethanone 4-Hydroxyacetophenone 4'-Hydroxyacetophenone p-Hydroxyacetophenone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.548 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
Piceol is used in the synthesis of several pharmaceutical drugs including octopamine, sotalol, bamethan, and dyclonine.
Piceol can be used to make acetaminophen by oxime formation with hydroxylamine and subsequent Beckmann rearrangement in acid.[4]
Anticonvulsants are also possible by Mannich reaction:[5]
Metabolism
Diprenylated derivatives of piceol can be isolated from Ophryosporus macrodon.[6]
4-Hydroxyacetophenone monooxygenase is an enzyme that transforms piceol into O-acetylhydroquinone. This enzyme is found in Pseudomonas fluorescens.
See also
- Paroxypropione, where the acetyl group is replaced by a propionyl group.
- Apocynin
References
- Løkke, H. (1990). "Picein and piceol concentrations in Norway spruce". Ecotoxicology and Environmental Safety. 19 (3): 301–9. doi:10.1016/0147-6513(90)90032-z. PMID 2364913.
- Münzenberger, Babette; Heilemann, Jürgen; Strack, Dieter; Kottke, Ingrid; Oberwinkler, Franz (1990). "Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce". Planta. 182 (1): 142–8. doi:10.1007/BF00239996. PMID 24197010.
- Løkke, Hans (1990). "Picein and piceol concentrations in Norway spruce". Ecotoxicology and Environmental Safety. 19 (3): 301–309. doi:10.1016/0147-6513(90)90032-Z. PMID 2364913.
- U.S. Patent 4,524,217
- Keshari, Amit K.; Tewari, Aseem; Verma, Shweta S.; Saraf, Shailendra K. (2017). "Novel Mannich-bases as Potential Anticonvulsants: Syntheses, Characterization and Biological Evaluation". Central Nervous System Agents in Medicinal Chemistry. 17 (3). doi:10.2174/1871524917666170717113524. ISSN 1871-5249.
- Sigstad, Elizabeth; Catalán, César A.N.; Diaz, Jesús G.; Herz, Werner (1993). "Diprenylated derivatives of p-hydroxyacetophenone from Ophryosporus macrodon". Phytochemistry. 33: 165–169. doi:10.1016/0031-9422(93)85415-N.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.