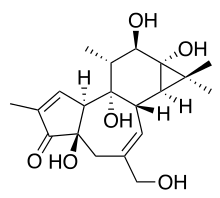
Phorbol
Phorbol is a natural, plant-derived organic compound. It is a member of the tigliane family of diterpenes. Phorbol was first isolated in 1934 as the hydrolysis product of croton oil, which is derived from the seeds of the purging croton, Croton tiglium.[2][3][4][5][6] The structure of phorbol was determined in 1967.[7][8] Various esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C.[9] They mimic diacylglycerols, glycerol derivatives in which two hydroxyl groups have reacted with fatty acids to form esters. The most common and potent phorbol ester is 12-O-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in contexts such as models of carcinogenesis.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1aR,1bS,4aR,7aS,9bS,8R,9R,9aS)-4a,7b,9,9a-Tetrahydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-5H-cyclopropa[3,4]benzo[1,2-e]azulen-5-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.162.035 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H28O6 | |
| Molar mass | 364.438 g·mol−1 |
| Melting point | 250 to 251 °C (482 to 484 °F; 523 to 524 K) |
| Soluble in DMSO (25mg/ml), 100% ethanol (25mg/ml), acetone, ether or dimethyl formamide; almost insoluble in aqueous buffers. | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
History and source
Phorbol is a natural product found in many plants, especially those of the Euphorbiaceae and Thymelaeaceae families.[10][11] Phorbol is the active constituent of the highly toxic New World tropical manchineel or beach apple, Hippomane mancinella.[12] It is very soluble in most polar organic solvents, as well as in water. In the manchineel, this leads to an additional exposure risk during rain, where liquid splashing from an undamaged tree may also be injurious. Contact with the tree or consumption of its fruit can lead to symptoms such as severe pain and swelling.[13][14]
The purging croton, Croton tiglium, is the source of croton oil from which phorbol was initially isolated. Its seeds and oil have been used for hundreds of years in traditional medicine, generally as a purgative, and the seeds were mentioned in Chinese herbal texts 2000 years ago.[15] The purgative effects of the oil are largely attributed to the high percentage of phorbol esters contained in the oil. Phorbol was isolated from C. tiglium seeds in 1934.[2][3][4][5][6] The structure of the compound was determined in 1967,[7][8] and a total synthesis was described in 2015.[16]
Mechanism of action
Phorbol derivatives work primarily by interacting with protein kinase C (PKC), although they can interact with other phospholipid membrane receptors.[17] The esters bind to PKC in a similar way to its natural ligand, diacylglycerol, and activate the kinase.[18] Diacylglycerol is degraded quickly by the body, allowing PKC to be reversibly activated. When phorbol esters bind to the receptor, they are not degraded as efficiently by the body, leading to constitutively active PK.[17] PKC is involved in a number of important cell signaling pathways. Thus, phorbol ester exposure can show a wide range of results.
The main results of phorbol exposure are tumor promotion and inflammatory response. Although phorbol is not a carcinogen itself, it greatly enhances the action of other substances and promotes tumor proliferation. PKC is a key component in biological pathways controlling cell growth and differentiation. When phorbol esters bind to PKC, cell proliferation pathways are activated. This effect greatly promotes tumors when the cells are exposed to even a sub-carcinogenic amount of a substance.[17] PKC is also involved in activation of inflammation pathways such as the NF-κB pathway. Thus, exposure to phorbol products can induce an inflammatory response in tissues.[19] Symptoms can include edema and pain, especially to the skin and mucus membranes.[10] While phorbol itself does not have irritant activity, nearly all phorbol esters are highly irritant, with a wide range of half-maximal inhibitory concentration (IC50) values.[10] The median lethal dose (LD50) of phorbol esters for male mice was found to be about 27 mg/kg, with the mice showing hemorrhage and congestion of pulmonary blood vessels, as well as lesions throughout the body.[18]
Total synthesis
A total synthesis of enantiopure phorbol was developed in 2015. While this synthesis will not replace natural isolation products, it will enable researchers to create phorbol analogs for use in research, especially creating phorbol derivatives that can be evaluated for anti-cancer activity.[16] Previously, the difficulty with synthesizing phorbol had been creating C–C bonds, especially in the six-membered ring at the top of the molecule. This synthesis starts from (+)-3-carene, and uses a series of 19 steps to eventually create (+)-phorbol.[20][21][16]
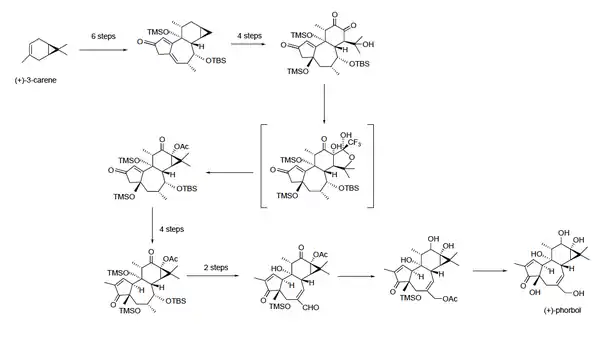 Overview of the complete synthesis of (+)-phorbol starting with (+)-3-carene
Overview of the complete synthesis of (+)-phorbol starting with (+)-3-carene
Uses in biomedical research
Because of their mechanism of action, phorbol esters can be used to study tumor proliferation and pain response. TPA is most commonly used in the laboratory to induce a cellular response. For example, TPA can be used to measure response to pain and test compounds that may mitigate the inflammatory response.[22] TPA and other phorbol esters can also be used to induce tumor formation and to study mechanism of action.[10] TPA, together with ionomycin, can also be used to stimulate T-cell activation, proliferation, and cytokine production, and is used in protocols for intracellular staining of these cytokines.
Possible and purported medicinal uses
The phorbol ester tigilanol tiglate reportedly has in vitro anti-cancer, antiviral, and antibacterial activities.[10] The phorbol derivatives in croton oil are used in folk medicine, with purported purgative, counter-irritant, or anthelmintic activities.[23]
Further reading
- Goel, Gunjan; Makkar, Harinder P.S.; Francis, George; Becker, Klaus (July 2007). "Phorbol Esters: Structure, Biological Activity, and Toxicity in Animals". International Journal of Toxicology. 26 (4): 279–288. CiteSeerX 10.1.1.320.6537. doi:10.1080/10915810701464641. PMID 17661218. S2CID 11550625. Retrieved 27 October 2023.
- Kikkawa, Ushio (June 2019) [November 2018]. "The Story of PKC: A Discovery Marked by Unexpected Twists and Turns". IUBMB Life. 71 (6): 697–705. doi:10.1002/iub.1963. Retrieved 27 October 2023.
References
- Merck Index, 11th Edition, 7306
- Flaschenträger B; v. Wolffersdorff R (1934). "Über den Giftstoff des Crotonöles. 1. Die Säuren des Crotonöles". Helvetica Chimica Acta. 17 (1): 1444–1452. doi:10.1002/hlca.193401701179.
- Flaschenträger B, Wigner G (1942). "Über den Giftstoff des Crotonöles. V. Die Gewinnung von Crotonharz, Dünnem Öl und Phorbol aus dem Crotonöl durch Alkoholyse". Helvetica Chimica Acta. 25 (3): 569–581. doi:10.1002/hlca.19420250315.
- Kauffmann T, Neumann H, Lenhardt K (1959). "Zur Konstitution des Phorbols, I. Über die reduzierende Gruppe des Phorbols". Chemische Berichte. 92 (8): 1715–1726. doi:10.1002/cber.19590920802.
- Kauffmann T, Eisinger A, Jasching W, Lenhardt K (1959). "Zur Konstitution des Phorbols, I. Über die reduzierende Gruppe des Phorbols". Chemische Berichte. 92 (8): 1727–1738. doi:10.1002/cber.19590920803.
- Tseng SS, van Duuren BL, Solomon JJ (1977). "Synthesis of 4aα-Phorbol 9-Myristate 9a-Acetate and Related Esters". J. Org. Chem. 42 (33): 3645–3649. doi:10.1021/jo00443a002. PMID 915585.
- Hecker E; Bartsch H; Bresch H; Gschwendt M; Härle B; Kreibich G; Kubinyi H; Schairer HU; v. Szczepanski C; Thielmann HW (1967). "Structure and Stereochemistry of the Tetracyclic Diterpene Phorbol from Croton tiglium L". Tetrahedron Letters. 8 (33): 3165–3170. doi:10.1016/S0040-4039(01)89890-7.
- Pettersen RC, Ferguson G, Crombie L, Games ML, Pointer DJ (1967). "The Structure and Stereochemistry of Phorbol, Diterpene Parent of Co-carcinogens of Croton Oil". Chem. Commun. 1967 (14): 716–717. doi:10.1039/C19670000716.
- Blumberg PM (1988). "Protein Kinase C as the Receptor for the Phorbol Ester Tumor Promoters: Sixth Rhoads Memorial Award Lecture" (PDF). Cancer Res. 48 (1): 1–8. PMID 3275491.
- Wang, Xiao-Yang; Liu, Li-Ping; Qin, Guo-Wei; Kang, Ting-Guo (2015). "Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families". Chemical Reviews. 115 (9): 2975–3011. doi:10.1021/cr200397n. PMID 25906056.
- Beutler, John A.; Alvarado, Ada Belinda; McCloud, Thomas G. (1989). "Distribution of phorbol ester bioactivity in the euphorbiaceae". Phytotherapy Research. 3 (5): 188–192. doi:10.1002/ptr.2650030507. S2CID 85408071.
- Adolf, W; Hecker, E (1984). "On the active principles of the spurge family. X. Skin irritants, cocarcingoens, and cryptic carcinogens from the latex of the manchineel tree". J Nat Prod. 47 (3): 482–496. doi:10.1021/np50033a015. PMID 6481361.
- Strickland, Nicola H (August 12, 2000). "Eating a manchineel "beach apple"". BMJ. 321 (7258): 428. doi:10.1136/bmj.321.7258.428. PMC 1127797. PMID 10938053.
- Blue, Lauren M; Sailing, Christopher; DeNapoles, Christopher; Fondots, Jordan; Johnson, Edward S (2011). "Manchineel Dermatitis in North American Students in the Caribbean". Journal of Travel Medicine. 18 (6): 422–424. doi:10.1111/j.1708-8305.2011.00568.x. PMID 22017721.
- Zhang, Xiao-Long; Wang, Lun; Li, Fu; Yu, Kai; Wang, Ming-Kui (2013). "Cytotoxic Phorbol Esters of Croton tiglium". Journal of Natural Products. 13 (5): 858–864. doi:10.1021/np300832n. PMID 23701597.
- Kawamura, Shuhei; Chu, Hang; Felding, Jakob; Baran, Phil S. (2016). "Nineteen-step total synthesis of (+)-phorbol". Nature. 532 (7597): 90–3. Bibcode:2016Natur.532...90K. doi:10.1038/nature17153. PMC 4833603. PMID 27007853..
- Goel, Gunjan; Makkar, Harinder P.S.; Francis, George; Becker, Klaus (July 2007). "Phorbol Esters: Structure, Biological Activity, and Toxicity in Animals". International Journal of Toxicology. 26 (4): 279–288. CiteSeerX 10.1.1.320.6537. doi:10.1080/10915810701464641. PMID 17661218. S2CID 11550625. Retrieved 27 October 2023.
- Li, Cai-Yan; Devappa, Rakshit K; Liu, Jian-Xin; Lv, Jian-Min; Makkar, HPS; Becker, K (February 2010). "Toxicity of Jatropha curcas phorbol esters in mice". Food and Chemical Toxicology. 48 (2): 620–625. doi:10.1016/j.fct.2009.11.042. PMID 19944127.
- Moscat, Jorge; Diaz-Meco, María T; Rennert, Paul (January 2003). "NF-κB activation by protein kinase C isoforms and B-cell function". EMBO Reports. 4 (1): 31–36. doi:10.1038/sj.embor.embor704. PMC 1315804. PMID 12524517.
- Wender, Paul A.; Kogen, Hiroshi; Lee, Hee Yoon; Munger, John D.; Wilhelm, Robert S.; Williams, Peter D. (1989). "Studies on tumor promoters. 8. The synthesis of phorbol". Journal of the American Chemical Society. 111 (24): 8957. doi:10.1021/ja00206a050.
- Wender, Paul A.; Rice, Kenneth D.; Schnute, Mark E. (1997). "The First Formal Asymmetric Synthesis of Phorbol". Journal of the American Chemical Society. 119 (33): 7897. doi:10.1021/ja9706256.
- Medeiros, Rodrigo; Otuki, Michel F; Avellar, Maria Christina W; Calixto, João (March 2007). "Mechanisms underlying the inhibitory actions of the pentacyclic triterpene α-amyrin in the mouse skin inflammation induced by phorbol ester 12-O-tetradecanoylphorbol-13-acetate". European Journal of Pharmacology. 22 (2–3): 227–235. doi:10.1016/j.ejphar.2006.12.005.
- Pal, Prince Kumar; Nandi, Manmath Kumar; Singh, Narendra Kumar (Jan–Mar 2014). "Detoxification of Croton tiglium L. seeds by Ayurvedic process of Śodhana". Ancient Science of Life. 33 (3): 157–161. doi:10.4103/0257-7941.144619. PMC 4264303. PMID 25538350.
External links
- Phorbols at the U.S. National Library of Medicine Medical Subject Headings (MeSH)