Phenylene group
In organic chemistry, the phenylene group (−C6H4−) is based on a di-substituted benzene ring (arylene). For example, poly(p-phenylene) is a polymer built up from para-phenylene repeating units.[1] The phenylene group has three structural isomers, based on which hydrogens are substituted: para-phenylene, meta-phenylene, and ortho-phenylene.
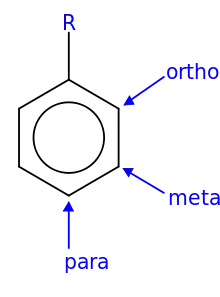
Isomer naming convention

The chemical structure of the para-phenylene group.
References
- p. C-9, Section 11.6, Handbook of Chemistry and Physics, 62nd Edition, 1981-1982, CRC Press
Wikimedia Commons has media related to Phenylene group.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.