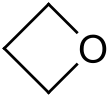
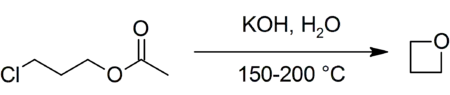Oxetane
Oxetane, or 1,3-propylene oxide, is a heterocyclic organic compound with the molecular formula C
3H
6O, having a four-membered ring with three carbon atoms and one oxygen atom.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxetane[1] | |||
| Systematic IUPAC name
1,3-Epoxypropane Oxacyclobutane | |||
| Other names
1,3-Propylene oxide Trimethylene oxide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 102382 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.241 | ||
| EC Number |
| ||
| 239520 | |||
PubChem CID |
|||
| UNII | |||
| UN number | 1280 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.08 g/mol | ||
| Density | 0.8930 g/cm3 | ||
| Melting point | −97 °C (−143 °F; 176 K) | ||
| Boiling point | 49 to 50 °C (120 to 122 °F; 322 to 323 K) | ||
Refractive index (nD) |
1.3895 at 25°C | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H225, H302, H312, H332 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P312, P322, P330, P363, P370+P378, P403+P235, P501 | |||
| Flash point | −28.3 °C; −19.0 °F; 244.8 K (NTP, 1992) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
The term "an oxetane" or "oxetanes" refer to any organic compound containing the oxetane ring.
Production
A typical well-known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150 °C:[2]
Yield of oxetane made this way is c. 40%, as the synthesis can lead to a variety of by-products including water, potassium chloride, and potassium acetate.
Another possible reaction to form an oxetane ring is the Paternò–Büchi reaction. The oxetane ring can also be formed through diol cyclization as well as through decarboxylation of a six-membered cyclic carbonate.
Taxol
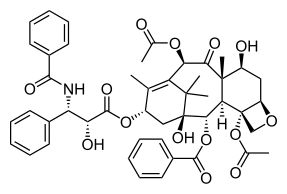
Paclitaxel (Taxol) is an example of a natural product containing an oxetane ring. Taxol has become a major point of interest among researchers due to its unusual structure and success in the involvement of cancer treatment.[3] The attached oxetane ring is an important feature that is used for the binding of microtubules in structure activity; however little is known about how the reaction is catalyzed in nature, which creates a challenge for scientists trying to synthesize the product.[3]
Derivatives
More than a hundred different oxetanes have been synthesized.[4] Functional groups can be added into any desired position in the oxetane ring, including fully fluorinated (perfluorinated) and fully deuterated analogues. Major examples are listed in table below.
| Name | Structure | Abbreviation | Boiling point, Bp [°C] |
|---|---|---|---|
| 3,3-Bis(chloromethyl)oxetane | oxetane.svg.png.webp) |
BCMO | 198[5] |
| 3,3-Bis(azidomethyl)oxetane | oxetane.svg.png.webp) |
BAMO | 165[6] |
| 2-Methyloxetane | 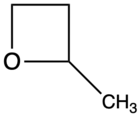 |
2MOX | 60[4] |
| 3-Methyloxetane |  |
3MOX | 67[4] |
| 3-Azidooxetane |  |
AZOX | 122[7] |
| 3-Nitrooxetane | 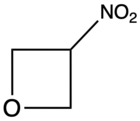 |
NIOX | 195[8] |
| 3,3-Dimethyloxetane | 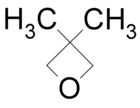 |
DMOX | 80[4] |
| 3,3-Dinitrooxetane | 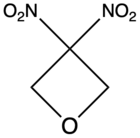 |
DNOX | – |
See also
- β-Propiolactone or 2-oxetanone.
- 3-Oxetanone
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- C. R. Noller (1955). "Trimethylene Oxide". Organic Syntheses. 29: 92.; Collective Volume, vol. 3, p. 835
- Willenbring, Dan; Tantillo, Dean J. (April 2008). "Mechanistic possibilities for oxetane formation in the biosynthesis of Taxol's D ring". Russian Journal of General Chemistry. 78 (4): 723–731. doi:10.1134/S1070363208040336. S2CID 98056619.
- Goethals, Lucien; Sioen, Philip (2000). "Dialogos. Lucien Goethals in gesprek met Philip Sioen". Revue belge de Musicologie / Belgisch Tijdschrift voor Muziekwetenschap. 54: 49–71. doi:10.2307/3686877. ISSN 0771-6788. JSTOR 3686877.
- "78-71-7 CAS MSDS (3,3-BIS(CHLOROMETHYL)OXETANE) Melting Point Boiling Point Density CAS Chemical Properties". www.chemicalbook.com. Retrieved 2022-12-28.
- Akhtar, Tauseef; Berger, Ronald; Marine, Joseph E; Daimee, Usama A; Calkins, Hugh; Spragg, David (2020-08-13). "Cryoballoon Ablation of Atrial Fibrillation in Octogenarians". Arrhythmia & Electrophysiology Review. 9 (2): 104–107. doi:10.15420/aer.2020.18. ISSN 2050-3377. PMC 7491081. PMID 32983532.
- Baum, Kurt; Berkowitz, Phillip T.; Grakauskas, Vytautas; Archibald, Thomas G. (September 1983). "Synthesis of electron-deficient oxetanes. 3-Azidooxetane, 3-nitrooxetane, and 3,3-dinitrooxetane". The Journal of Organic Chemistry. 48 (18): 2953–2956. doi:10.1021/jo00166a003. ISSN 0022-3263.
- "3-Nitrooxetane | C3H5NO3 | ChemSpider". www.chemspider.com. Retrieved 2022-12-28.