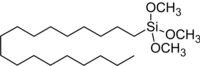
Octadecyltrimethoxysilane
Octadecyltrimethoxysilane (OTMS) is an organosilicon compound. This colorless liquid is used for preparing hydrophobic coatings and self-assembled monolayers. It is sensitive toward water, irreversibly degrading to a siloxane polymer.[2] It places a C18H39SiO3 "cap" on oxide surfaces. The formation of OTMS monolayers is used for converting hydrophilic surfaces to hydrophobic surfaces, e.g. for use in certain areas of nanotechnology and analytical chemistry.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Trimethoxy(octadecyl)silane | |
| Other names
n-Octadecyltrimethoxysilane Trimethoxyoctadecylsilane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | OTMS |
| 5791830 | |
| ChemSpider | |
| ECHA InfoCard | 100.019.400 |
| EC Number |
|
| MeSH | n-Octadecyltrimethoxysilane |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H46O3Si | |
| Molar mass | 374.681 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.883 g cm−3 |
| Melting point | 16 to 17 °C (61 to 63 °F; 289 to 290 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
Refractive index (nD) |
1.438-1.44 |
| Hazards[1] | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "Octadecyltrimethoxysilane". pubchem.ncbi.nlm.nih.gov. Retrieved 5 December 2021.
- P. Fontaine; F. Rondelez (1995). J. Daillant; P. Guenoun; C. Marques; P. Muller; J. Tran Thanh Van (eds.). Kinetics of Polymerisation in Langmuir Monolayers of n-Alkyltrimethoxysilane.
{{cite book}}:|work=ignored (help)
Further reading
- Hild, R; David, C; Müller, H. U; Völkel, B; Kayser, D. R; Grunze, M (1998). "Formation and Characterization of Self-assembled Monolayers of Octadecyltrimethoxysilane on Chromium: Application in Low-Energy Electron Lithography". Langmuir. 14 (2): 342–346. doi:10.1021/la970438l.
- Vidon, S; Leblanc, R. M (1998). "Langmuir Study of Octadecyltrimethoxysilane Behavior at the Air−Water Interface". The Journal of Physical Chemistry B. 102 (7): 1279–1286. doi:10.1021/jp973334s.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
